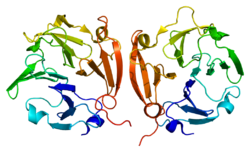MMP9
Matrix metalloproteinase-9 (MMP-9), also known as 92 kDa type IV collagenase, 92 kDa gelatinase or gelatinase B (GELB), is a matrixin, a class of enzymes that belong to the zinc-metalloproteinases family involved in the degradation of the extracellular matrix. In humans the MMP9 gene[5] encodes for a signal peptide, a propeptide, a catalytic domain with inserted three repeats of fibronectin type II domain followed by a C-terminal hemopexin-like domain.[6]
Function
[edit]Proteins of the matrix metalloproteinase (MMP) family are involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, angiogenesis, bone development, wound healing, cell migration, learning and memory, as well as in pathological processes, such as asthma, arthritis, intracerebral hemorrhage,[7] and metastasis.[8] Most MMPs are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. The enzyme encoded by this gene degrades type IV and V collagens and other extracellular matrix proteins.[9] Studies in rhesus monkeys suggest that the enzyme is involved in IL-8-induced mobilization of hematopoietic progenitor cells from bone marrow, and murine studies suggest a role in tumor-associated tissue remodeling.[5]
Thrombospondins, intervertebral disc proteins, regulate interaction with matrix metalloproteinases (MMPs) 2 and 9, which are key effectors of ECM remodeling.[10]
Neutrophil action
[edit]MMP9, along with elastase, appears to be a regulatory factor in neutrophil migration across the basement membrane.[11]
MMP9 plays several important functions within neutrophil action, such as degrading extracellular matrix, activation of IL-1β, and cleavage of several chemokines.[12] In a mouse model, MMP9 deficiency resulted in resistance to endotoxin shock, suggesting that MMP9 is important in sepsis.[13]
Angiogenesis
[edit]MMP9 may play an important role in angiogenesis and neovascularization. For example, MMP9 appears to be involved in the remodeling associated with malignant glioma neovascularization.[14] It is also a key regulator of growth plate formation- both growth plate angiogenesis and the generation of hypertrophic chondrocytes. Knock-out models of MMP9 result in delayed apoptosis, vascularization, and ossification of hypertrophic chondrocytes.[15] Lastly, there is significant evidence that Gelatinase B is required for the recruitment of endothelial stem cells, a critical component of angiogenesis [16]
Wound repair
[edit]MMP9 is greatly upregulated during human respiratory epithelial healing.[17] Using a MMP9 deficient mouse model, it was seen that MMP9 coordinated epithelial wound repair and deficient mice were unable to remove the fibrinogen matrix during wound healing.[18] When interacting with TGF-ß1, Gelatinase B also stimulates collagen contraction, aiding in wound closure.[19]
Structure
[edit]
MMP9 is synthesized as preproenzyme of 707 amino-acid residues, including a 19 amino acid signal peptide and secreted as an inactive pro-MMP. The human MMP9 proenzyme consists of five domains. The amino-terminal propeptide, the zinc-binding catalytic domain and the carboxyl-terminal hemopexin-like domain are conserved. Its primary structure comprises several domain motifs. The propeptide domain is characterized by a conserved PRCGVPD sequence. The Cys within this sequence is known as the “cysteine switch”. It ligates the catalytic zinc to maintain the enzyme in an inactive state.[6]

Activation is achieved through an interacting protease cascade involving plasmin and stromelysin 1 (MMP-3). Plasmin generates active MMP-3 from its zymogen. Active MMP-3 cleaves the propeptide from the 92-kDa pro-MMP-9, yielding an 82-kDa enzymatically active enzyme.[21] In the active enzyme a substrate, or a fluorogenic activity probe.,[20] replaces the propeptide in the enzyme active site where it is cleaved. The catalytic domain contains two zinc and three calcium atoms. The catalytic zinc is coordinated by three histidines from the conserved HEXXHXXGXXH binding motif. The other zinc atom and the three calcium atoms are structural. A conserved methionine, which forms a unique “Met-turn” structure categorizes MMP9 as a metzincin.[22] Three type II fibronectin repeats are inserted in the catalytic domain, although these domains are omitted in most crystallographic structures of MMP9 in complex with inhibitors. The active form of MMP9 also contains a C-terminal hemopexin-like domain. This domain is ellipsoidal in shape, formed by four β-propeller blades and an α-helix. Each blade consists of four antiparallel β-strands arranged around a funnel-like tunnel that contains two calcium and two chloride ions.[23] The hemopexin domain is important to facilitate the cleavage of triple helical interstitial collagens. .
Clinical significance
[edit]MMP9 has been found to be associated with numerous pathological processes, including cancer, placental malaria, immunologic and cardiovascular diseases.
Arthritis
[edit]Elevated MMP9 levels can be found in the cases of rheumatoid arthritis[24] and focal brain ischemia.[25]
Cancer
[edit]One of MMP9's most widely associated pathologies is the relationship to cancer, due to its role in extracellular matrix remodeling and angiogenesis. For example, its increased expression was seen in a metastatic mammary cancer cell line.[26] Gelatinase B plays a central role in tumor progression, from angiogenesis, to stromal remodeling, and ultimately metastasis.[27] However, because of its physiologic function, it may be difficult to leverage Gelatinase B inhibition into cancer therapy modalities. However, Gelatinase B has been investigated in tumor metastasis diagnosis- Complexes of Gelatinase B/Tissue Inhibitors of Metalloproteinases are seen to be increased in gastrointestinal cancer and gynecologic malignancies [28]
MMPs such as MMP9 can be involved in the development of several human malignancies, as degradation of collagen IV in basement membrane and extracellular matrix facilitates tumor progression, including invasion, metastasis, growth and angiogenesis.[29]
Cardiovascular
[edit]MMP9 levels increase with the progression of idiopathic atrial fibrillation.[30]
MMP9 has been found to be associated with the development of aortic aneurysms,[31] and its disruption prevents the development of aortic aneurysms.[32] Doxycycline suppresses the growth of aortic aneurysms in animal models through its inhibition of MMP9 reduces aortic inflammation in humans.[33]
Pregnancy-associated malaria (Placental malaria)
[edit]A study on Ghanaian population showed that MMP-9 single nucleotide polymorphism 1562 C > T (rs3918242) was protective against placental malaria which suggests a possible role of MMP-9 in susceptibility to malaria.[34]
Dry eye
[edit]Dry eye patients, especially with meibomian gland dysfunction exhibit higher levels of MMP-9.[35]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000100985 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000017737 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b "Matrix metallopeptidase 9 (gelatinase B, 92kDa gelatinase, 92kDa type IV collagenase)".
- ^ a b Nagase H, Woessner JF (July 1999). "Matrix metalloproteinases". The Journal of Biological Chemistry. 274 (31): 21491–4. doi:10.1074/jbc.274.31.21491. PMID 10419448.
- ^ Wang J, Tsirka SE (July 2005). "Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage". Brain. 128 (Pt 7): 1622–33. doi:10.1093/brain/awh489. PMID 15800021.
- ^ Vandooren J, Van den Steen PE, Opdenakker G (2013). "Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade". Critical Reviews in Biochemistry and Molecular Biology. 48 (3): 222–72. doi:10.3109/10409238.2013.770819. PMID 23547785. S2CID 33781725.
- ^ Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G (December 2002). "Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9)". Critical Reviews in Biochemistry and Molecular Biology. 37 (6): 375–536. doi:10.1080/10409230290771546. PMID 12540195. S2CID 35833950.
- ^ Hirose Y, Chiba K, Karasugi T, Nakajima M, Kawaguchi Y, Mikami Y, Furuichi T, Mio F, Miyake A, Miyamoto T, Ozaki K, Takahashi A, Mizuta H, Kubo T, Kimura T, Tanaka T, Toyama Y, Ikegawa S (May 2008). "A functional polymorphism in THBS2 that affects alternative splicing and MMP binding is associated with lumbar-disc herniation". American Journal of Human Genetics. 82 (5): 1122–9. doi:10.1016/j.ajhg.2008.03.013. PMC 2427305. PMID 18455130.
- ^ Delclaux C, Delacourt C, D'Ortho MP, Boyer V, Lafuma C, Harf A (March 1996). "Role of gelatinase B and elastase in human polymorphonuclear neutrophil migration across basement membrane". American Journal of Respiratory Cell and Molecular Biology. 14 (3): 288–95. doi:10.1165/ajrcmb.14.3.8845180. PMID 8845180.
- ^ Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, Proost P, Van Damme J (June 2001). "Gelatinase B functions as regulator and effector in leukocyte biology". Journal of Leukocyte Biology. 69 (6): 851–9. doi:10.1189/jlb.69.6.851. PMID 11404367. S2CID 15851048.
- ^ Dubois B, Starckx S, Pagenstecher A, Oord Jv, Arnold B, Opdenakker G (August 2002). "Gelatinase B deficiency protects against endotoxin shock". European Journal of Immunology. 32 (8): 2163–71. doi:10.1002/1521-4141(200208)32:8<2163::AID-IMMU2163>3.0.CO;2-Q. PMID 12209628.
- ^ Forsyth PA, Wong H, Laing TD, Rewcastle NB, Morris DG, Muzik H, Leco KJ, Johnston RN, Brasher PM, Sutherland G, Edwards DR (April 1999). "Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas". British Journal of Cancer. 79 (11–12): 1828–35. doi:10.1038/sj.bjc.6690291. PMC 2362801. PMID 10206300.
- ^ Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z (May 1998). "MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes". Cell. 93 (3): 411–22. doi:10.1016/s0092-8674(00)81169-1. PMC 2839071. PMID 9590175.
- ^ Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S (May 2002). "Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand". Cell. 109 (5): 625–37. doi:10.1016/s0092-8674(02)00754-7. PMC 2826110. PMID 12062105.
- ^ Buisson AC, Zahm JM, Polette M, Pierrot D, Bellon G, Puchelle E, Birembaut P, Tournier JM (February 1996). "Gelatinase B is involved in the in vitro wound repair of human respiratory epithelium". Journal of Cellular Physiology. 166 (2): 413–26. doi:10.1002/(sici)1097-4652(199602)166:2<413::aid-jcp20>3.0.co;2-a. PMID 8592002. S2CID 24996115.
- ^ Mohan R, Chintala SK, Jung JC, Villar WV, McCabe F, Russo LA, Lee Y, McCarthy BE, Wollenberg KR, Jester JV, Wang M, Welgus HG, Shipley JM, Senior RM, Fini ME (January 2002). "Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration". The Journal of Biological Chemistry. 277 (3): 2065–72. doi:10.1074/jbc.m107611200. PMID 11689563.
- ^ Kobayashi T, Kim H, Liu X, Sugiura H, Kohyama T, Fang Q, Wen FQ, Abe S, Wang X, Atkinson JJ, Shipley JM, Senior RM, Rennard SI (June 2014). "Matrix metalloproteinase-9 activates TGF-β and stimulates fibroblast contraction of collagen gels". American Journal of Physiology. Lung Cellular and Molecular Physiology. 306 (11): L1006-15. doi:10.1152/ajplung.00015.2014. PMC 4042193. PMID 24705725.
- ^ a b Tranchant I, Vera L, Czarny B, Amoura M, Cassar E, Beau F, Stura EA, Dive V (March 2014). "Halogen bonding controls selectivity of FRET substrate probes for MMP-9". Chemistry & Biology. 21 (3): 408–13. doi:10.1016/j.chembiol.2014.01.008. PMID 24583051.
- ^ Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP (May 1999). "Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion". The Journal of Biological Chemistry. 274 (19): 13066–76. doi:10.1074/jbc.274.19.13066. PMID 10224058.
- ^ Bode W, Gomis-Rüth FX, Stöckler W (September 1993). "Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the 'metzincins'". FEBS Letters. 331 (1–2): 134–40. doi:10.1016/0014-5793(93)80312-I. PMID 8405391. S2CID 27244239.
- ^ Gomis-Rüth FX, Gohlke U, Betz M, Knäuper V, Murphy G, López-Otín C, Bode W (December 1996). "The helping hand of collagenase-3 (MMP-13): 2.7 A crystal structure of its C-terminal haemopexin-like domain". Journal of Molecular Biology. 264 (3): 556–66. doi:10.1006/jmbi.1996.0661. PMID 8969305.
- ^ Gruber BL, Sorbi D, French DL, Marchese MJ, Nuovo GJ, Kew RR, Arbeit LA (February 1996). "Markedly elevated serum MMP-9 (gelatinase B) levels in rheumatoid arthritis: a potentially useful laboratory marker". Clinical Immunology and Immunopathology. 78 (2): 161–71. doi:10.1006/clin.1996.0025. PMID 8625558.
- ^ Clark AW, Krekoski CA, Bou SS, Chapman KR, Edwards DR (November 1997). "Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia". Neuroscience Letters. 238 (1–2): 53–6. doi:10.1016/s0304-3940(97)00859-8. PMID 9464653. S2CID 916260.
- ^ Morini M, Mottolese M, Ferrari N, Ghiorzo F, Buglioni S, Mortarini R, Noonan DM, Natali PG, Albini A (August 2000). "The alpha 3 beta 1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (MMP-9) activity". International Journal of Cancer. 87 (3): 336–42. doi:10.1002/1097-0215(20000801)87:3<336::aid-ijc5>3.3.co;2-v. PMID 10897037.
- ^ Farina AR, Mackay AR (January 2014). "Gelatinase B/MMP-9 in Tumour Pathogenesis and Progression". Cancers. 6 (1): 240–96. doi:10.3390/cancers6010240. PMC 3980597. PMID 24473089.
- ^ Zucker S, Lysik RM, DiMassimo BI, Zarrabi HM, Moll UM, Grimson R, Tickle SP, Docherty AJ (August 1995). "Plasma assay of gelatinase B: tissue inhibitor of metalloproteinase complexes in cancer". Cancer. 76 (4): 700–8. doi:10.1002/1097-0142(19950815)76:4<700::aid-cncr2820760426>3.0.co;2-5. PMID 8625169. S2CID 41700819.
- ^ Groblewska M, Siewko M, Mroczko B, Szmitkowski M (April 2012). "The role of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in the development of esophageal cancer". Folia Histochemica et Cytobiologica. 50 (1): 12–9. doi:10.5603/fhc.2012.0002. PMID 22532131.
- ^ Li M, Yang G, Xie B, Babu K, Huang C (February 2014). "Changes in matrix metalloproteinase-9 levels during progression of atrial fibrillation". The Journal of International Medical Research. 42 (1): 224–30. doi:10.1177/0300060513488514. PMID 24345823.
- ^ Newman KM, Ogata Y, Malon AM, Irizarry E, Gandhi RH, Nagase H, Tilson MD (August 1994). "Identification of matrix metalloproteinases 3 (stromelysin-1) and 9 (gelatinase B) in abdominal aortic aneurysm". Arteriosclerosis and Thrombosis. 14 (8): 1315–20. doi:10.1161/01.atv.14.8.1315. PMID 8049193.
- ^ Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW (June 2000). "Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms". The Journal of Clinical Investigation. 105 (11): 1641–9. doi:10.1172/jci8931. PMC 300851. PMID 10841523.
- ^ Lindeman JH, Abdul-Hussien H, van Bockel JH, Wolterbeek R, Kleemann R (April 2009). "Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells". Circulation. 119 (16): 2209–16. doi:10.1161/CIRCULATIONAHA.108.806505. PMID 19364980.
- ^ Apoorv TS, Babu PP, Meese S, Gai PP, Bedu-Addo G, Mockenhaupt FP (July 2015). "Matrix metalloproteinase-9 polymorphism 1562 C > T (rs3918242) associated with protection against placental malaria". The American Journal of Tropical Medicine and Hygiene. 93 (1): 186–8. doi:10.4269/ajtmh.14-0816. PMC 4497894. PMID 26013370.
- ^ Messmer, Elisabeth M.; von Lindenfels, Victoria; Garbe, Alexandra; Kampik, Anselm (November 2016). "Matrix Metalloproteinase 9 Testing in Dry Eye Disease Using a Commercially Available Point-of-Care Immunoassay". Ophthalmology. 123 (11): 2300–2308. doi:10.1016/j.ophtha.2016.07.028. PMID 27665213.
Further reading
[edit]- Nagase H, Woessner JF (July 1999). "Matrix metalloproteinases". The Journal of Biological Chemistry. 274 (31): 21491–4. doi:10.1074/jbc.274.31.21491. PMID 10419448.
- Zhao X, Wu T, Chang CF, Wu H, Han X, Li Q, Gao Y, Li Q, Hou Z, Maruyama T, Zhang J, Wang J (May 2015). "Toxic role of prostaglandin E2 receptor EP1 after intracerebral hemorrhage in mice". Brain, Behavior, and Immunity. 46: 293–310. doi:10.1016/j.bbi.2015.02.011. PMC 4422065. PMID 25697396.
- Starckx S, Van den Steen PE, Wuyts A, Van Damme J, Opdenakker G (February 2002). "Neutrophil gelatinase B and chemokines in leukocytosis and stem cell mobilization". Leukemia & Lymphoma. 43 (2): 233–41. doi:10.1080/10428190290005982. PMID 11999552. S2CID 940921.
- Wu H, Zhang Z, Li Y, Zhao R, Li H, Song Y, Qi J, Wang J (October 2010). "Time course of upregulation of inflammatory mediators in the hemorrhagic brain in rats: correlation with brain edema". Neurochemistry International. 57 (3): 248–53. doi:10.1016/j.neuint.2010.06.002. PMC 2910823. PMID 20541575.
- Bischof P, Meisser A, Campana A (2002). "Control of MMP-9 expression at the maternal-fetal interface". Journal of Reproductive Immunology. 55 (1–2): 3–10. doi:10.1016/S0165-0378(01)00142-5. PMID 12062817.
- St-Pierre Y, Van Themsche C, Estève PO (September 2003). "Emerging features in the regulation of MMP-9 gene expression for the development of novel molecular targets and therapeutic strategies". Current Drug Targets. Inflammation and Allergy. 2 (3): 206–15. doi:10.2174/1568010033484133. PMID 14561155. S2CID 453825.
- Wu H, Wu T, Hua W, Dong X, Gao Y, Zhao X, Chen W, Cao W, Yang Q, Qi J, Zhou J, Wang J (March 2015). "PGE2 receptor agonist misoprostol protects brain against intracerebral hemorrhage in mice". Neurobiology of Aging. 36 (3): 1439–50. doi:10.1016/j.neurobiolaging.2014.12.029. PMC 4417504. PMID 25623334.
- Lee JM, Yin K, Hsin I, Chen S, Fryer JD, Holtzman DM, Hsu CY, Xu J (March 2005). "Matrix metalloproteinase-9 in cerebral-amyloid-angiopathy-related hemorrhage". Journal of the Neurological Sciences. 229–230: 249–54. doi:10.1016/j.jns.2004.11.041. PMID 15760647. S2CID 21791281.
- Nair RR, Boyd DD (November 2005). "Expression cloning of novel regulators of 92 kDa type IV collagenase expression". Biochemical Society Transactions. 33 (Pt 5): 1135–6. doi:10.1042/BST20051135. PMID 16246065.
- Wu H, Zhang Z, Hu X, Zhao R, Song Y, Ban X, Qi J, Wang J (June 2010). "Dynamic changes of inflammatory markers in brain after hemorrhagic stroke in humans: a postmortem study". Brain Research. 1342: 111–7. doi:10.1016/j.brainres.2010.04.033. PMC 2885522. PMID 20420814.
- Wu H, Wu T, Han X, Wan J, Jiang C, Chen W, Lu H, Yang Q, Wang J (January 2017). "Cerebroprotection by the neuronal PGE2 receptor EP2 after intracerebral hemorrhage in middle-aged mice". Journal of Cerebral Blood Flow and Metabolism. 37 (1): 39–51. doi:10.1177/0271678X15625351. PMC 5363749. PMID 26746866.
- Ram M, Sherer Y, Shoenfeld Y (July 2006). "Matrix metalloproteinase-9 and autoimmune diseases". Journal of Clinical Immunology. 26 (4): 299–307. doi:10.1007/s10875-006-9022-6. PMID 16652230. S2CID 19619963.