Lyman series
In physics, the Lyman series is the series of transitions and resulting emission lines of the hydrogen atom as an electron goes from n ≥ 2 to n = 1 (where n is the principal quantum number referring to the energy level of the electron). The transitions are named sequentially by Greek letters: from n = 2 to n = 1 is called Lyman-alpha, 3 to 1 is Lyman-beta, 4 to 1 is Lyman-gamma, etc.
The first line in the ultraviolet spectrum of the Lyman series was first discovered in 1906 by Harvard physicist Theodore Lyman, who was studying the ultraviolet spectrum of electrically excited hydrogen gas. The rest of the lines of the spectrum were discovered by Lyman from 1906-1914.
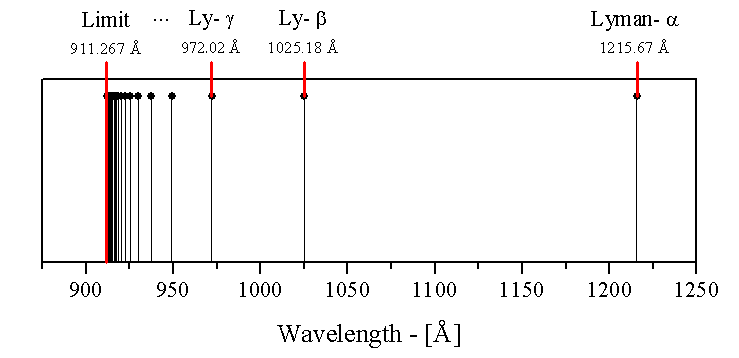
The spectrum of radiation emitted by hydrogen is non-continuous. Here is an illustration of the first series of hydrogen emission lines:
Historically, explaining the nature of this spectrum was a considerable problem in physics. Nobody could predict the wavelengths of the hydrogen lines until Johannes Rydberg came up with an empirical formula that solved the problem in 1888. A schoolteacher at the time, he managed to find a formula to match the known emission lines and predict those which were not yet discovered. The Rydberg formula was:
Where n is a natural number greater or equal than 2 (i.e. n = 2,3,4,...).
Therefore, the lines seen in the image are the wavelengths corresponding to n=2 on the left, to n= on the right (there are infinitely many spectral lines, but they become very dense as they approach to n=, so only some of the first lines and the last one appear).
The wavelengths (nm) in the Lyman series are:
- 2-1 -- 121.5
- 3-1 -- 102.5
- 4-1 --- 97.2
- 5-1 --- 94.9
- 6-1 --- 93.7
- 7-1 --- 93.0
- 8-1 --- 92.6
- 9-1 --- 92.3
- 10-1 -- 92.1
- 11-1 -- 91.9
- Limit: 91.15 nm
Later, when Niels Bohr produced his Bohr atom theory, the reason why the spectral lines fit Rydberg's formula was explained. Bohr found that the electron bound to the hydrogen atom must have quantized energy levels described by the following formula:
According to Bohr's third assumption, whenever an electron falls from an initial energy level() to a final energy level(), the atom must emit radiation with a wavelength of:
There is also a more comfortable notation when dealing with energy in units of electronvolts and wavelengths in units of angstroms:
Replacing the energy in the above formula with the expression for the energy in the hydrogen atom where the initial energy corresponds to energy level n and the final energy corresponds to energy level m:
where R is the same constant Rydberg found. It is easy then to see the connection between what Bohr found and what Rydberg found. Replacing m by 1 we get:
which is exactly Rydberg's formula. Therefore, each wavelength of the emission lines corresponds to an electron dropping from a certain energy level (greater than 1) to the first energy level.
The series is named after its discoverer, Theodore Lyman.



![{\displaystyle E_{n}=-{{me^{4}} \over {2\left(4\pi \varepsilon _{0}\hbar \right)^{2}}}{1 \over n^{2}}=-{13.6 \over n^{2}}[{\mbox{eV}}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/22b490298f7ef1141e1e2c37ba12a04e7c7a4ea2)