Helium compounds
Helium is the smallest and the lightest noble gas and one of the most unreactive elements, so it was commonly considered that helium compounds cannot exist at all, or at least under normal conditions.[1] Helium's first ionization energy of 24.57 eV is the highest of any element.[2] Helium has a complete shell of electrons, and in this form the atom does not readily accept any extra electrons nor join with anything to make covalent compounds. The electron affinity is 0.080 eV, which is very close to zero.[2] The helium atom is small with the radius of the outer electron shell at 0.29 Å.[2] Helium is a very hard atom with a Pearson hardness of 12.3 eV.[3] It has the lowest polarizability of any kind of atom, however, very weak van der Waals forces exist between helium and other atoms. This force may exceed repulsive forces, so at extremely low temperatures helium may form van der Waals molecules. Helium has the lowest boiling point (4.2 K) of any known substance.
Repulsive forces between helium and other atoms may be overcome by high pressures. Helium has been shown to form a crystalline compound with sodium under pressure. Suitable pressures to force helium into solid combinations could be found inside planets. Clathrates are also possible with helium under pressure in ice, and other small molecules such as nitrogen.
Other ways to make helium reactive are: to convert it into an ion, or to excite an electron to a higher level, allowing it to form excimers. Ionised helium (He+), also known as He II, is a very high energy material able to extract an electron from any other atom. He+ has an electron configuration like hydrogen, so as well as being ionic it can form covalent bonds. Excimers do not last for long, as the molecule containing the higher energy level helium atom can rapidly decay back to a repulsive ground state, where the two atoms making up the bond repel. However, in some locations such as helium white dwarfs, conditions may be suitable to rapidly form excited helium atoms. The excited helium atom has a 1s electron promoted to 2s. This requires 1,900 kilojoules (450 kcal) per gram of helium, which can be supplied by electron impact, or electric discharge.[4] The 2s excited electron state resembles that of the lithium atom.
Known solid phases
[edit]Most solid combinations of helium with other substances require high pressure. Helium does not bond with the other atoms, but the substances can have a well defined crystal structure.[citation needed]
Disodium helide
[edit]Disodium helide (Na2He) is a compound of helium and sodium that is stable at high pressures above 113 gigapascals (1,130,000 bar). Disodium helide was first predicted[5] using USPEX code and was first synthesised in 2016.[2][6] It was predicted to be thermodynamically stable over 160 GPa and dynamically stable over 100 GPa. Na2He has a cubic crystal structure, resembling fluorite. At 300 GPa the edge of a unit cell of the crystal has a = 3.95 Å. Each unit cell contains four helium atoms on the centre of the cube faces and corners, and eight sodium atoms at coordinates a quarter cell in from each face. Double electrons (2e−) are positioned on each edge and the centre of the unit cell.[7] Each pair of electrons is spin paired. The presence of these isolated electrons makes this an electride. The helium atoms do not participate in any bonding. However the electron pairs can be considered as an eight-centre two-electron bond.[2] Disodium helide is predicted to be an insulator and transparent.[2]
Silicates
[edit]Helium was first observed to enter into a silicate in 2007. The mineral melanophlogite is a natural silica clathrate (clathrasil) that normally would contain carbon dioxide, methane or nitrogen. When compressed with helium, a new clathrate forms. This has a much higher bulk modulus, and resists amorphization. Helium was taken up around 17 GPa, enlarging the unit cell, and given off again when pressure dropped to 11 GPa.[8]
Cristobalite He II (SiO2He) is stable between 1.7 and 6.4 GPa. It has a rhombohedral space group R-3c with unit cell dimensions a = 9.080 Å, α = 31.809° and V = 184.77 Å3 at 4 GPa.[9]
Cristobalite He I (SiO2He) can be formed under higher helium pressures over 6.4 GPa. It has a monoclinic space group P21/C with unit cell dimensions a = 8.062 Å, b = 4.797 Å, c = 9.491 Å, β = 120.43° and V = 316.47 Å3 at 10 GPa.[10]
Helium penetrates into fused silica at high pressure, reducing its compressibility.[11]
Chibaite, another natural silica clathrate has its structure penetrated by helium under pressures higher than 2.5 GPa. The presence of guest hydrocarbons does not prevent this happening. Neon requires a higher pressure, 4.5 GPa to penetrate, and unlike helium shows hysteresis.[12] Linde-type A zeolites are also rendered less compressible when penetrated by helium between 2 and 7 GPa.[13]
Arsenolite helium inclusion compound
[edit]Arsenolite helium inclusion compound As4O6·2He is stable from pressures over 3 GPa and up to at least 30 GPa.[14] Arsenolite is one of the softest and most compressible minerals.[15] Helium prevents amorphization that would otherwise occur in arsenolite under pressure.[16] The solid containing helium is stronger and harder, with a higher sound velocity than plain arsenolite.[17] The helium that is included into the crystal causes a more uniform stress on the As4O6 molecules. No actual bond is formed from arsenic to helium despite the lone pairs of electrons available.[18] The diffusion of helium into arsenolite is a slow process taking days at a pressure around 3 GPa. However, if the pressure on the crystal is too high (13 GPa) helium penetration does not take place, as the gaps between arsenolite molecules become too small.[18] Neon does not diffuse into arsenolite.[18]
Perovskites
[edit]Helium can be inserted into the A sites of negative thermal expansion perovskites that otherwise have defects at the A site. At room temperature and 350 MPa helium is included into CaZrF6 to expand its unit cell yielding HeCaZrF6. About half of the A sites are filled by helium atoms. This substance loses helium over several minutes on depressurisation at ambient temperature, but below 130 K it retains helium when depressurised.[19] At 1 GPa all the A sites are filled by helium, yielding He2CaZrF6.[20]
Formates
[edit]Under pressure helium penetrates dimethylammonium iron formate (CH3)2NH2Fe(HCOO)3. It affects this by causing a change to a monoclinic ordered state at a lower pressure (around 4 GPa) than if helium were absent.[21]
Small molecule
[edit]He(N2)11 is a van der Waals compound with hexagonal crystals. At 10 GPa the unit cell of 22 nitrogen atoms has a unit cell volume of 558 Å3, and about 512 Å3 at 15 GPa. These sizes are around 10 Å3 smaller than the equivalent amount of solid δ-N2 nitrogen at these pressures. The substance is made by compressing nitrogen and helium in a diamond anvil cell.[22][23]
NeHe2 has a crystal structure of hexagonal MgZn2 type at 13.7 GPa. The unit cell has dimensions a = 4.066 Å, c = 6.616 Å; and at 21.8 GPa, a = 3.885 Å, c = 6.328 Å. There are four atoms in each unit cell. It melts at 12.8 GPa and 296 K,[24] stable to over 90 GPa.[25]
Clathrates
[edit]Helium clathrates only form under pressure. With ice II at pressures between 280 and 480 MPa a solid helium hydrate with He:H2O ratio of 1:6 exists.[26] Another clathrate with a water to helium ratio of 2.833 has been made in the SII clathrate structure. It has two different cages in the ice, the small one can contain one helium atom, and the large can contain four atoms. It was produced from neon clathrate that lost its neon, and then replaced by helium at 141 K and 150 MPa[27] Other helium hydrates with the ice-Ih, ice-Ic 1:1, and ice-Ic 2:1 He to H2O ratio have been predicted.[26] These could exist in planets like Neptune or Uranus.[27] Helium clathrate hydrates should be similar to hydrogen clathrate due to the similar size of the hydrogen molecule.[27]
Helium may enter into crystals of other molecular solids under pressure to alter their structure and properties. For example, with chlorpropamide over 0.3 GPa in helium changes to a monoclinic structure, and yet another structural form at 1.0 GPa.[28]
Fullerites
[edit]Helium can form intercalation compounds with the fullerites, including buckminsterfullerene C60 and C70. In solid C60 there are spaces between the C60 balls, either tetrahedral or octahedral in shape. Helium can diffuse into the solid fullerite even at one atmosphere pressure. Helium enters the lattice in two stages. The first rapid stage takes a couple of days, and expands the lattice by 0.16% (that is 2.2 pm) filling the larger octahedral sites. The second stage takes thousands of hours to absorb more helium and expands the lattice twice as much again (0.32%) filling the tetrahedral sites. However the solid C60•3He is not stable and loses helium on a timescale of 340 hours when not under a helium atmosphere. When the helium intercalated fullerite is cooled, it has an orientational phase transition that is 10 K higher than for pure solid C60. The actual discontinuous change in volume at that point is smaller, but there are more rapid changes near the transition temperature, perhaps due to varying occupancy of the voids by helium.[29][30]
Endohedral
[edit]Helium atoms can be trapped inside molecular cages such as the fullerenes He@C60, He@C70, He2@C60 and He2@C70 have all been made using compressed helium and fullerenes.[31] When using only pressure and heat, the yield is quite low, under 1%. However, by breaking and reforming the carbon ball, much higher concentrations of He@C60 or He@C70 can be made. High-performance liquid chromatography can concentrate the helium containing material. HeN@C60 and HeN@C70 have also been made. These have a lower symmetry due to the two atoms being trapped together in the same cavity. This causes ESR line broadening.[32]
Dodecahedrane can trap helium from a helium ion beam to yield He@C20H20.
Other cage like inorganic or organic molecules may also trap helium, for example C8He with He inside a cube,[33] or He@Mo6Cl8F6.[34]
Impurity helium condensates
[edit]Impurity helium condensates (IHCs) (or impurity helium gels)[35] are deposited as a snow-like gel in liquid helium when various atoms or molecules are absorbed on the surface of superfluid helium. Atoms can include H, N, Na, Ne, Ar, Kr, Xe, alkalis or alkaline earths. The impurities form nanoparticle clusters coated with localised helium held by van der Waals force. Helium atoms are unable to move towards or away from the impurity, but perhaps can move perpendicularly around the impurity.[36] The snow like solid is structured like an aerogel. When free atoms are included in the condensate a high energy density can be achieved, up to 860 J cm−1 or 5 kJ g−1.[37] These condensates were first investigated as a possible rocket fuel.[38] The mixtures are given a notation involving square brackets so that [N]/[He] represents a nitrogen atom impurity in helium.[citation needed]
[N]/[He] atomic nitrogen impurity helium is produced when a radio frequency discharge in a nitrogen helium mixture is absorbed into superfluid helium, it can have up to 4% nitrogen atoms included.[39] The substance resembles crumbly snow and condenses and settles from the liquid helium.[39] It also contains variable proportions of N2 molecules.[39] This substance is a high energy solid, with as much power as conventional explosives. When it is heated above 2.19 K (the lambda point of helium), the solid decomposes and explodes.[39] This substance is not a true compound, but more like a solid solution.[36] E. B. Gordon et al. suggested that this material may exist in 1974.[39] The localised helium shells around an individual atom are termed van der Waals spheres.[39] However the idea that the nitrogen atoms are dispersed in the helium has been replaced by the concept of nitrogen atoms attached to the surface of clusters of nitrogen molecules. The energy density of the solid can be increased by pressing it.[40]
Other inert gas impurity helium condensates can also be made from a gas beam into superfluid helium.[41] [Ne]/[He] decomposes at 8.5 K with release of heat and formation of solid neon. Its composition approximates NeHe16.
[Ar]/[He] contains 40–60 helium atoms per argon atom.[42]
[Kr]/[He] contains 40–60 helium atoms per krypton atom[42] and is stable up to 20 K.[37]
[Xe]/[He] contains 40–60 helium atoms per xenon atom.[42]
[N2]/[He] contains 12—17 He atoms per N2 molecule.[42] It is stable up to 13 K[37]
[N]/[Ne]/[He] Formed from a gas beam generated from a radio-frequency electric discharge in mixtures of neon, nitrogen and helium blown into superfluid He. Additional inert gas stabilises more nitrogen atoms. It decomposes around 7 K with a blue green light flash.[41] Excited nitrogen atoms in the N(2D) state can be relative long lasting, up to hours, and give off a green luminescence.[41]
[H2]/[He], or [D2]/[He] when dihydrogen or dideuterium is absorbed into superfluid helium, filaments are formed. When enough of these form, the solid resembles cotton, rather than snow.[43] Using H2 results in the product floating and stopping further production, but with deuterium, or a half-half mixture, it can sink and accumulate.[37] Atomic hydrogen in impurity helium decays fairly rapidly due to quantum tunneling (H + H → H2). Atomic deuterium dimerises slower (D + D → D2), but reacts very quickly with any diprotium present. (D + H2 → HD + H).[37] Atomic hydrogen solids are further stabilised by other noble gases such as krypton.[44][45][46] Lowering temperatures into the millikelvin range can prolong the lifetime of atomic hydrogen condensates.[38] Condensates containing heavy water or deuterium are under investigation for the production of ultracold neutrons.[35] Other impurity gels have been investigated for producing ultracold neutrons include CD4 (deuterated methane) and C2D5OD. (deuterated ethanol)[47]
The water-helium condensate [H2O]/[He] contains water clusters of several nanometers in diameter, and pores from 8 to 800 nm.[48]
Oxygen O2 impurity helium contains solid oxygen clusters from 1 to 100 nm.[49]
Impurity solid helium
[edit]Introducing impurities into solid helium yields a blue solid that melts at a higher temperature than pure He.[50] For cesium the absorption has a peak at 750 nm, and for rubidium, maximal absorption is at 640 nm. These are due to metal clusters with diameters of 10 nm or so. However the low concentration of clusters in this substance should not be sufficient to solidify helium as the amount of metal in the solid is less than billionth that of the impurity helium condensate solids, and liquid helium does not "wet" cesium metal. The solid is possibly due to helium snowballs attached to Cs+ (or Rb+) ions.[50] The snowball is a shell that contains helium atoms solidified in particular positions around the ion. The helium atoms are immobilized in the snowball by polarization. Neutral metallic atoms in liquid helium are also surrounded by a bubble caused by electron repulsion. They have typical sizes ranging from 10 to 14 Å diameter.[51] Free electrons in liquid helium are enclosed in a bubble 17 Å in diameter. Under 25 atmosphere pressure an electron bubble reduces to 11 Å.[52]
Solid solution
[edit]Helium can dissolve to a limited extent in hot metal, with concentration proportional to pressure. At atmospheric pressure, 500 °C bismuth can absorb 1 part in a billion; at 649 °C lithium can take 5 parts per billion; and at 482 °C potassium can take 2.9 parts per million (all atom fractions).[53] In nickel there can be 1 in 1010 atoms, and in gold 1 in 107. The supposition is that the higher the melting point the less helium can be dissolved. However, when a liquid metal is quenched, higher concentrations of helium can be left dissolved. So cooled liquid steel can have one part per million of helium. In order to get a helium atom into a metal lattice, a hole has to be formed. The energy to make that hole in the metal is basically the heat of solution.[54]
Nanowires
[edit]Gold, copper, rubidium, caesium, or barium atoms evaporated into liquid helium form spiderweb-like structures.[55] Rhenium produces nano flakes. Molybdenum, tungsten, and niobium produce thin nanowires with diameters of 20, 25 and 40 Å.[56] When platinum, molybdenum or tungsten is evaporated into liquid helium, nanoclusters are first formed, accompanied by high temperature thermal emission pulse, above the melting point of the metals. In superfluid helium, these clusters migrate to the vortices and weld together to yield nanowires once the clusters are mostly solid. In higher temperature liquid helium, larger clusters of metal are formed instead of wires. The metal vapours can only penetrate about 0.5 mm into liquid helium.[57] Indium, tin, lead and nickel produce nanowires about 80 Å in diameter.[58] These same four metals also produce smooth spheres about 2 μm across that explode when examined with an electron microscope.[59] Copper, permalloy, and bismuth also make nanowires.[60]
Two-dimensional ionic crystal
[edit]Helium II ions (He+) in liquid helium when attracted by an electric field can form a two-dimensional crystal at temperatures below 100 mK. There are about half a trillion ions per square meter just below the surface of the helium. Free electrons float above the helium surface.[61]
Known van der Waals molecules
[edit]- LiHe[62]
- Dihelium
- Trihelium
- Ag3He[63]
- HeCO is weakly bound by van der Waals forces. It is potentially important in cold interstellar media as both CO and He are common.[64]
- CF4He and CCl4He both exist.[65]
- HeI2 can be formed by supersonic expansion of high pressure helium with a trace of iodine into a vacuum. It was the first known triatomic helium van der Waals molecule. It can be detected by fluorescence. HeI2 has a similar optical spectrum to I2, except that the bands and lines are shifted to form two extra series. One series is blueshifted by between 2.4 and 4.0 cm−1, and the other between 9.4 and 9.9 cm−1. The two series may be due to different amounts of vibration in the He–I bond. The lines are narrow indicating that the molecules in their excited vibrational state have a long lifetime.[66]
- Na2He molecules can form on the surface of helium nanodroplets.[67]
- NOHe[68]
Known ions
[edit]Helium has the highest ionisation energy, so a He+ ion will strip electrons off any other neutral atom or molecule. However it can also then bind to the ion produced. The He+ ion can be studied in gas, or in liquid helium. Its chemistry is not completely trivial. For example, He+ can react with SF6 to yield SF+
6 or SF+
5 and atomic fluorine.[69]
Ionised clusters
[edit]He+
2 was predicted to exist by Linus Pauling in 1933. It was discovered when doing mass spectroscopy on ionised helium. The dihelium cation is formed by an ionised helium atom combining with a helium atom: He+ + He → He+
2.[70]
The diionised dihelium He2+
2 (1Σ+
g) is in a singlet state. It breaks up He2+
2 → He+ + He+ releasing 200 kcal/mol of energy. It has a barrier to decomposition of 35 kcal/mol and a bond length of 0.70 Å.[70]
The trihelium cation He+
3[71] is in equilibrium with He+
2 between 135 and 200K.[72]
Helium hydride
[edit]The helium hydride ion HeH+ has been known since 1925.[70] The protonated dihelium ion He2H+ can be formed when the dihelium cation reacts with dihydrogen: He+
2 + H2 → He2H+ + H. This is believed to be a linear molecule.[70] Larger protonated helium cluster ions exist HenH+ with n from 3 to 14. He6H+ and He13H+ appear to be more common. These can be made by reacting H+
2 or H+
3 with gaseous helium.[70]
HeH2+ is unstable in its ground state. But when it is excited to the 2pσ state the molecule is bound with an energy of 20 kcal/mol. This doubly charged ion has been made by accelerating the helium hydride ion to 900 keV, and firing it into argon. It only has a short life of 4 ns.[70]
H2He+ has been made and could occur in nature via H2 + He+ → H2He+.[70]
H3He+
n exists for n from 1 to over 30, and there are also clusters with more hydrogen atoms and helium.[73]
Noble gas
[edit]Noble gas cluster ions exist for different noble gases. Singly charged cluster ions containing xenon exist with the formula HenXe+
m, where n and m ≥ 1.[74]
Many different HenKr+ exist with n between 1 and 17, with higher values possible. HenKr+
2 and HenKr+
3 also exist for many values of n. He12Kr+
2 and He12Kr+
3 ions are common. These singly charged cluster ions can be made from krypton in helium nanodroplets subject to vacuum ultraviolet radiation.[74]
The Ar+ argon ion can form many different sized clusters with helium ranging from HeAr+ to He50Ar+, but the most common clusters are He12Ar+ and smaller. These clusters are made by capturing an argon atom in a liquid helium nanodroplet, and then ionising with high speed electrons. He+ is formed, which can transfer charge to argon and then form a cluster ion when the rest of the droplet evaporates.[75]
NeHe+
n can be made by ultraviolet photoionisation. Clusters only contain one neon atom. The number of helium atoms can vary from 1 to 23, but NeHe+
4 and NeHe+
8 are more likely to be observed.[74]
Doubly charged ions of helium with noble gas atoms also exist including ArHe2+, KrHe2+, and XeHe2+.[76]
Metals
[edit]Various metal-helium ions are known.
Alkali metal helide ions are known for all the alkalis. The molecule ground state for the diatomic ions is in the X1Σ+ state. The bond length gets bigger as the periodic table is descended with lengths of 1.96, 2.41, 2.90, 3.10, and 3.38 Å for Li+He, Na+He, K+He, Rb+He, and Cs+He. The dissociation energies are 1.9, 0.9, 0.5, 0.4 and 0.3 kcal/mol, showing bond energy decreases. When the molecule breaks up the positive charge is never on the helium atom.[70]
When there are many helium atoms around, alkali metal ions can attract shells of helium atoms. Clusters can be formed from absorbing metal into helium droplets. The doped droplets are ionised with high speed electrons. For sodium clusters appear with the formula Na+Hen with n from 1 to 26. Na+He is the most common, but Na+He2 is very close in abundance. Na+He8 is much more abundant than clusters with more helium. Na+
2Hen with n from 1 to 20 also appears. Na+
3Hen with small n is also made. For potassium, K+Hen with n up to 28, and K+
2Hen for n from 1 to 20 is formed. K+He and K+He2 are both common, and K+He12 is a bit more commonly formed than other similar sized clusters.[77] Cesium and rubidium cations also form clusters with helium.[77]
Other known metal-helium ions include Cr+He, Co+He, Co+He3, Ni+He, and Ni+He3.[70] PtHe2+;[78][79] formed by high electric field off platinum surface in helium,[76] VHe2+,[76] HeRh2+ is decomposed in high strength electric field,[80][81] Ta2+He, Mo2+He, W2+He, Re2+He, Ir2+He, Pt2+He2, W3+He2, W3+He3, and W3+He4.[70]
Nonmetals
[edit]HeN+
2 can form at around 4 K from an ion beam of N+
2 into cold helium gas.[82] The energy needed to break up the molecule is 140 cm−1 which is quite a bit stronger than the van der Waals neutral molecules. HeN+
2 is tough enough to have several vibrational, bending and rotational states.[83] HenN+
2 with n from 2 to 6 have been made by shooting electrons at a supersonically expanding mix of nitrogen and helium.[70]
C60He+ is formed by irradiating C60 with 50eV electrons and then steering ions into cold helium gas. C60He+
2 is also known.[84]
He(OH)+ has been detected, although it is not produced when HTO (tritiated water) decays.[70]
He
n(CO)+
has been detected for values of n from 1 to 12. Also CH3He+, OCHHe+ and NH2He+ have been detected.[70]
Young and Coggiola claimed to make HeC+ by an electric discharge off graphite into helium.[85]
When tritium substituted methane (CH3T) decays, CH3He+ is produced in a very small amount.[86]
The helium formyl cation, HeHCO+ is a linear molecule. It has a vibrational frequency red shifted 12.4 cm−1 compared to HCO+. It can be considered as a deenergized protonation reaction intermediate for the HeH+ + CO → HCO+ + He.[83] HeHCO+ can be produced by a supersonic expansion of a gas mixture of He, CO, and H2, which is hit by a cross beam of electrons. CO and H2 are only supplied at 1% of the helium.[83]
The HeHN+
2 molecule is linear. The He-H bondlength is 1.72 Å. It has an infrared band, due to B-H stretching, with a base at 3158.42 cm−1.[83][87] The binding energy is 378 cm−1 in the 000 vibrational state, and 431 cm−1 in the 100 vibrational state.[88] He2HN+
2 is also known. One helium atom is linked to a hydrogen, and the other is less tightly bound.[88]
H2O+, H2OSF5+, SF5+ and SF6+ can form clusters with varying numbers of Helium atoms.[89]
Excimers
[edit]The He*
2 excimer is responsible for the Hopfield continuum. Helium also forms an excimer with barium, Ba+He*.[90]
Predicted compounds
[edit]Predicted solids
[edit]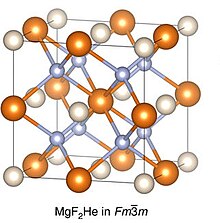
He(H2O)2 is predicted to form a solid with orthorhombic structure Ibam.[91]
Iron helide (FeHe) was early on claimed to have been found,[92] but the discovery was classified as an alloy.[53] Early studies predicted the FeHe exists as an interstitial compound under high pressure,[93] perhaps in dense planetary cores,[94] or, as suggested by Freeman Dyson, in neutron star crust material.[95] Recent density functional theory calculations predict the formation of FeHe compounds at pressures above about 4 TPa,[96] suggesting indeed that these compounds could be found inside giant planets, white dwarf stars, or neutron stars.
Na2HeO is predicted to have a similar structure to Na2He, but with oxygen atoms in the same position as the electron pair, so that it becomes O2−. It would be stable from 13 to 106 GPa.[2] This substance could be a way to store helium in a solid.[97]
La2/3-xLi3xTiO3He is a porous lithium ion conduction perovskite that can contain helium like a clathrate.[33]
Helium is predicted to be included under pressure in ionic compounds of the form A2B or AB2. These compounds could include Na2OHe, MgF2He (over 107 GPa) and CaF2He (30-110 GPa). Stabilisation occurs by the helium atom positioning itself between the two like charged ions, and partially shielding them from each other.[98]
Helium is predicted to form an inclusion compound with silicon, Si2He. This has a hexagonal lattice of silicon atoms with helium atoms lined up in the channels. It should be formed when liquid silicon is injected with helium at over 1GPa and cooled.[99]
Predicted van der Waals molecules
[edit]The beryllium oxide helium adduct, HeBeO is believed to be bonded much more strongly than a normal van der Waals molecule with about 5 kcal/mol of binding energy. The bond is enhanced by a dipole induced positive charge on beryllium, and a vacancy in the σ orbital on beryllium where it faces the helium.[100][101]
Variations on the beryllium oxide adduct include HeBe2O2,[101] RNBeHe including HNBeHe, CH3NBeHe,[101] CH4−xNBeHex, SiH4−xNBeHex, NH3−xNBeHex, PH3−xNBeHex, OH2−xNBeHex, SH2−xNBeHex,[102] and HeBe(C5H5)+.[103]
Hydridohelium fluoride HHeF is predicted to have a lifetime 157 femtoseconds 05 kcal/mol barrier[clarification needed].[104] The lifetime of the deuterium isotopomer is predicted to be much longer due to a greater difficulty of tunneling for deuterium.[105] This molecule's metastability is slated due to electrostatic attraction between HHe+ and F− which increases the barrier to an exothermic breakup.[100] Under pressures over 23 GPa HHeF should be stable.[106]
Calculations for coinage metal fluorides include HeCuF as stable,[104] HeAgF is unstable,[104] HeAuF is predicted,[104] and Ag3He with binding energy 1.4 cm−1,[107] Ag4He binding energy 1.85 cm−1, Au3He binding energy 4.91 cm−1,[107] and Au4He binding energy 5.87 cm−1[107]
HeNaO is predicted.
Calculation for binary van der Waals helium molecules include HeNe,
Li4He binding energy 0.008 cm−1, the Li3He is not stable.[107]
Na4He binding energy 0.03 cm−1, the Na3He is not stable.[107]
Cu3He binding energy 0.90 cm−1,[107]
O4He binding energy 5.83 cm−1,[107]
S4He binding energy 6.34 cm−1,[107]
Se4He binding energy 6.50 cm−1,[107]
F4He binding energy 3.85 cm−1,[107]
Cl4He binding energy 7.48 cm−1,[107]
Br4He binding energy 7.75 cm−1,[107]
I4He binding energy 8.40 cm−1,[107]
N4He binding energy 2.85 cm−1,[107]
P4He binding energy 3.42 cm−1,[107]
As4He binding energy 3.49 cm−1,[107]
Bi4He binding energy 33.26 cm−1,[107]
Si4He binding energy 1.95 cm−1,[107]
Ge4He binding energy 2.08 cm−1,[107]
CaH4He binding energy 0.96 cm−1,[107]
NH4He binding energy 4.42 cm−1,[107]
MnH4He binding energy 1.01 cm−1,[107]
YbF4He binding energy 5.57 cm−1[107]
I4
2He or I3
2He,[108]
Bonds are predicted to form to nickel with helium as a weak ligand in HeNiCO and HeNiN2.[100]
(HeO)(LiF)2 is predicted to form a planar metastable molecule.[109] 1-Tris(pyrazolyl)borate beryllium and 1-tris(pyrazolyl)borate magnesium are predicted to bind helium at low temperatures.[110] There is also a prediction of a He-O bond in a molecule with caesium fluoride or tetramethyl ammonium fluoride.[111]
LiHe2 is predicted to be in an Efimov state when excited.[112]
Predicted ions
[edit]
Many ions have been investigated theoretically to see if they could exist. Just about every diatomic cation with helium has been studied. For the diatomic dications, for stability the second ionisation level of the partner atom has to be below the first ionisation level of helium, 24.6 eV. For Li, F, and Ne the ground state is repulsive, so molecules will not form. For N and O the molecule would break up to release He+. However HeBe2+, HeB2+ and HeC2+ are predicted to be stable. Also second row elements from Na to Cl are predicted to have a stable HeX2+ ion.[70]
HeY3+ is predicted to be the lightest stable diatomic triply charged ion.[113] Other possibly thermochemically stable ions include HeZr3+, HeHf3+, HeLa3+, HeNd3+, HeCe3+, HePr3+, HePm3+, HeSm3+, HeGa3+, HeTb3+, HeDy3+, HeHo3+, HeEr3+, HeTm3+, and HeLu3+ where the third ionisation point is below that of helium.[70]
The positronium helide ion PsHe+ should be formed when positrons encounter helium.[114]
The Fluoroheliate FHeO− ion should be stable but salts like LiFHeO are not stable.[115][71]
- HHeCO+ theoretical[116]
- FHeS− is predicted to be stable.[117]
- FHeBN−
- HHeN2+ is unlikely to exist.[118]
- (HHe+)(OH2) is probably unstable.[119]
The lithium hydrohelide cation HLiHe+ is linear in theory. This molecular ion could exist with big bang nucleosynthesis elements.[120] Other hydrohelide cations that exist in theory are HNaHe+ sodium hydrohelide cation, HKHe+ potassium hydrohelide cation, HBeHe2+ beryllium hydrohelide cation, HMgHe2+ magnesium hydrohelide cation, and HCaHe2+ calcium hydrohelide cation.[120]
HeBeO+ is predicted to have a relatively high binding energy of 25 kcal mol−1.[121]
For negative ions the adduct is very weakly bound.[70] Those studied include HeCl−, HeBr−, HeF−, HeO− and HeS−.[71]
- FHeS−[71]
- FHeSe−[71]
- C7H6He2+[71]
- C7H6HeHe2+[71]
- FHeCC−[71]
- HHeOH+
2[71] - HHeBF+[71]
- HeNC+[71]
- HeNN+[71]
- HHeNN+ H-He 0.765 Å He-N bond length 2.077 Å. Decomposition barrier of 2.3 kJ/mol.[71]
HHeNH+
3 is predicted to have a C3v symmetry and a H-He bond length of 0.768 Å and He-N 1.830. The energy barrier against decomposition to ammonium is 19.1 kJ/mol with an energy release of 563.4 kJ/mol. Cleavage to helium hydride ion and ammonia is predicted to be endothermic, requiring 126.2 kJ/mol.[71]
Discredited or unlikely observations
[edit]Numerous researchers attempted to create chemical compounds of helium in the early part of the twentieth century.[122] In 1895 L. Troost and L. Ouvrard believed they had witnessed a reaction between magnesium vapour and helium (and also argon) due to the spectrum of helium disappearing from the tube they were passing it through.[123] In 1906, W. Ternant Cooke claimed to have noticed a reaction of helium with cadmium or mercury vapour by observing an increase in the density of the vapour. Zinc vapour did not react with helium.[124]
J. J. Manley claimed to have found gaseous mercury helide HeHg in 1925[125][126][127] HgHe10;[128][129] publishing the results in Nature, but then had trouble finding a stable composition, and eventually gave up.
Between 1925 and 1940 in Buenos Aires, Horacio Damianovich studied various metal–helium combinations including beryllium (BeHe), iron (FeHe), palladium (PdHe), platinum (Pt3He), bismuth, and uranium.[130][92] To make these substances, electrical discharges impacted helium into the surface of the metal.[4] Later these were demoted from the status of compounds, to that of alloys.[53]
Platinum helide, Pt3He was discredited by J. G. Waller in 1960.[131]
Palladium helide, PdHe is formed from tritium decay in palladium tritide, the helium (3He) is retained in the solid as a solution.
Boomer claimed the discovery of tungsten helide WHe2 as a black solid.[132] It is formed by way of an electric discharge in helium with a heated tungsten filament. When dissolved in nitric acid or potassium hydroxide, tungstic acid forms and helium escapes in bubbles. The electric discharge had a current of 5 mA and 1,000 V at a pressure between 0.05 and 0.5 mmHg for the helium. The process works slowly at 200 V. and 0.02 mmHg of mercury vapour accelerates tungsten evaporation by five times. The search for this was suggested by Ernest Rutherford. It was discredited by J. G. Waller in 1960.[131] Boomer also studied mercury, iodine, sulfur, and phosphorus combinations with helium. Mercury and iodine helium combinations decomposed around −70 °C[133] Sulfur and phosphorus helium combinations decomposed around −120 °C[133]
H. Krefft and R. Rompe claimed reactions between helium and sodium, potassium, zinc, rubidium, indium, and thallium.[137]
References
[edit]- ^ Cotton, F. Albert; Wilkinson, Geoffrey (1966). Advanced Inorganic Chemistry. John Wiley. pp. 140–141.
- ^ a b c d e f g Dong, Xiao; Oganov, Artem R. (25 April 2014). "Stable Compound of Helium and Sodium at High Pressure". Nature Chemistry. 9 (5): 440–445. arXiv:1309.3827. Bibcode:2017NatCh...9..440D. doi:10.1038/nchem.2716. PMID 28430195. S2CID 20459726.
- ^ Grochala, W. (1 January 2009). "On Chemical Bonding Between Helium and Oxygen" (PDF). Polish Journal of Chemistry. 83 (1): 87–122. Archived from the original (abstract) on 2 February 2017. Retrieved 17 May 2016.
- ^ a b Kana'an, Adli S.; Margrave, John L. (1964). "Chemical Reactions in Electrical Discharges". In Emeleus, H. J.; Sharpe, A. G. (eds.). Advances in Inorganic Chemistry and Radiochemistry volume 6. Cambridge, England: Academic Press. pp. 182–183. ISBN 9780080578552.
- ^ Saleh, Gabriele; Dong, Xiao; Oganov, Artem; Gatti, Carlo; Qian, Guang-rui; Zhu, Qiang; Zhou, Xiang-Feng; Wang, Hiu-tian (5 August 2014). "Stable Compound of Helium and Sodium at High Pressure". Acta Crystallographica Section A. 70 (a1): 440–445. arXiv:1309.3827. doi:10.1107/S2053273314093826. PMID 28430195.
- ^ Dong, Xiao; Oganov, Artem R.; Goncharov, Alexander F.; Stavrou, Elissaios; Lobanov, Sergey; Saleh, Gabriele; Qian, Guang-Rui; Zhu, Qiang; Gatti, Carlo; Deringer, Volker L.; Dronskowski, Richard; Zhou, Xiang-Feng; Prakapenka, Vitali B.; Konôpková, Zuzana; Popov, Ivan A.; Boldyrev, Alexander I.; Wang, Hui-Tian (6 February 2017). "A stable compound of helium and sodium at high pressure". Nature Chemistry. 9 (5): 440–445. arXiv:1309.3827. Bibcode:2017NatCh...9..440D. doi:10.1038/nchem.2716. PMID 28430195. S2CID 20459726.
- ^ Each face is shared by two cells, each edge is shared by four cells, and each corner is shared by eight cells.
- ^ Yagi, Takehiko; Iida, Etsuko; Hirai, Hisako; Miyajima, Nobuyoshi; Kikegawa, Takumi; Bunno, Michiaki (24 May 2007). "High-pressure behavior of a SiO2 clathrate observed by using various pressure media". Physical Review B. 75 (17): 174115. Bibcode:2007PhRvB..75q4115Y. doi:10.1103/PhysRevB.75.174115.
- ^ Matsui, M.; Sato, T.; Funamori, N. (2 January 2014). "Crystal structures and stabilities of cristobalite-helium phases at high pressures" (PDF). American Mineralogist. 99 (1): 184–189. Bibcode:2014AmMin..99..184M. doi:10.2138/am.2014.4637. S2CID 54034818.
- ^ Matsui, M.; Sato, T.; Funamori, N. (2 January 2014). "Crystal structures and stabilities of cristobalite-helium phases at high pressures". American Mineralogist. 99 (1): 184–189. Bibcode:2014AmMin..99..184M. doi:10.2138/am.2014.4637. S2CID 54034818.
- ^ Sato, Tomoko; Funamori, Nobumasa; Yagi, Takehiko (14 June 2011). "Helium penetrates into silica glass and reduces its compressibility". Nature Communications. 2: 345. Bibcode:2011NatCo...2..345S. doi:10.1038/ncomms1343. PMID 21673666.
- ^ Scheidl, K.S.; Effenberger, H.S.; Yagi, T.; Momma, K.; Miletich, R. (January 2019). "Transformation pathways and isothermal compressibility of a MTN-type clathrasil using penetrating and non-penetrating fluids". Microporous and Mesoporous Materials. 273: 73–89. doi:10.1016/j.micromeso.2018.06.033. S2CID 103129909.
- ^ Niwa, Ken; Tanaka, Tatsuya; Hasegawa, Masashi; Okada, Taku; Yagi, Takehiko; Kikegawa, Takumi (December 2013). "Pressure-induced noble gas insertion into Linde-type A zeolite and its incompressible behaviors at high pressure". Microporous and Mesoporous Materials. 182: 191–197. doi:10.1016/j.micromeso.2013.08.044.
- ^ Guńka, Piotr A.; Dziubek, Kamil F.; Gładysiak, Andrzej; Dranka, Maciej; Piechota, Jacek; Hanfland, Michael; Katrusiak, Andrzej; Zachara, Janusz (August 2015). "Compressed Arsenolite As4O6 and Its Helium Clathrate As4O6·2He". Crystal Growth & Design. 15 (8): 3740–3745. doi:10.1021/acs.cgd.5b00390.
- ^ Sans, Juan A.; Manjón, Francisco J.; Popescu, Catalin; Cuenca-Gotor, Vanesa P.; Gomis, Oscar; Muñoz, Alfonso; Rodríguez-Hernández, Plácida; Contreras-García, Julia; Pellicer-Porres, Julio; Pereira, Andre L. J.; Santamaría-Pérez, David; Segura, Alfredo (1 February 2016). "Ordered helium trapping and bonding in compressed arsenolite: Synthesis of As4O5•2He". Physical Review B. 93 (5): 054102. arXiv:1502.04279. Bibcode:2016PhRvB..93e4102S. doi:10.1103/PhysRevB.93.054102. hdl:10251/65644. S2CID 118635331.
- ^ Sans, Juan A.; Manjón, Francisco J.; Popescu, Catalin; Cuenca-Gotor, Vanesa P.; Gomis, Oscar; Muñoz, Alfonso; Rodríguez-Hernández, Plácida; Contreras-García, Julia; Pellicer-Porres, Julio; Pereira, Andre L. J.; Santamaría-Pérez, David; Segura, Alfredo (1 February 2016). "Ordered helium trapping and bonding in compressed arsenolite: Synthesis of". Physical Review B. 93 (5): 054102. Bibcode:2016PhRvB..93e4102S. doi:10.1103/PhysRevB.93.054102. hdl:10251/65644. S2CID 118635331.
- ^ Cuenca-Gotor, V. P.; Gomis, O.; Sans, J. A.; Manjón, F. J.; Rodríguez-Hernández, P.; Muñoz, A. (21 October 2016). "Vibrational and elastic properties of As4O6 and As4O6·2He at high pressures: Study of dynamical and mechanical stability". Journal of Applied Physics. 120 (15): 155901. Bibcode:2016JAP...120o5901C. doi:10.1063/1.4964875. hdl:10251/80142.
- ^ a b c Guńka, Piotr A.; Hapka, Michał; Hanfland, Michael; Dranka, Maciej; Chałasiński, Grzegorz; Zachara, Janusz (5 April 2018). "How and Why Does Helium Permeate Nonporous Arsenolite Under High Pressure?". ChemPhysChem. 19 (7): 857–864. doi:10.1002/cphc.201701156. PMID 29341365.
- ^ Hester, Brett R.; dos Santos, António M.; Molaison, Jamie J.; Hancock, Justin C.; Wilkinson, Angus P. (13 September 2017). "Synthesis of Defect Perovskites (He2–x□x)(CaZr)F6 by Inserting Helium into the Negative Thermal Expansion Material CaZrF6". Journal of the American Chemical Society. 139 (38): 13284–13287. doi:10.1021/jacs.7b07860. OSTI 1399917. PMID 28892378.
- ^ Lloyd, Anthony J.; Hester, Brett R.; Baxter, Samuel J.; Ma, Shangye; Prakapenka, Vitali B.; Tkachev, Sergey N.; Park, Changyong; Wilkinson, Angus P. (21 April 2021). "Hybrid Double Perovskite Containing Helium: [He 2 ] [CaZr]F 6". Chemistry of Materials. 33 (9): 3132–3138. doi:10.1021/acs.chemmater.0c04782. OSTI 1813113. S2CID 234814221.
- ^ Collings, Ines E.; Bykov, Maxim; Bykova, Elena; Hanfland, Michael; van Smaalen, Sander; Dubrovinsky, Leonid; Dubrovinskaia, Natalia (2018). "Disorder–order transitions in the perovskite metal–organic frameworks [(CH3)2NH2] [M(HCOO)3] at high pressure". CrystEngComm. 20 (25): 3512–3521. doi:10.1039/C8CE00617B. S2CID 103144439.
- ^ Vos, W. L.; Finger, L. W.; Hemley, R. J.; Hu, J. Z.; Mao, H. K.; Schouten, J. A. (2 July 1992). "A high-pressure van der Waals compound in solid nitrogen-helium mixtures". Nature. 358 (6381): 46–48. Bibcode:1992Natur.358...46V. doi:10.1038/358046a0. S2CID 4313676.
- ^ Li, Xiangdong; Su, Hao; Liang, Wentao; Zhou, Wenju; Rahman, Azizur; Xu, Zilong; Zhong, Cheng; Mai, Di; Dai, Rucheng; Gou, Huiyang; Wang, Zhongping; Zheng, Xianxu; Wu, Qiang; Zhang, Zengming (1 June 2022). "Inference of a "Hot Ice" Layer in Nitrogen-Rich Planets: Demixing the Phase Diagram and Phase Composition for Variable Concentration Helium–Nitrogen Mixtures Based on Isothermal Compression". The Journal of Physical Chemistry A. 126 (23): 3745–3757. Bibcode:2022JPCA..126.3745L. doi:10.1021/acs.jpca.2c02132. PMID 35648656. S2CID 249235942.
- ^ Loubeyre, Paul; Jean-Louis, Michel; LeToullec, René; Charon-Gérard, Lydie (11 January 1993). "High pressure measurements of the He–Ne binary phase diagram at 296 K: Evidence for the stability of a stoichiometric Ne(He)2 solid". Physical Review Letters. 70 (2): 178–181. Bibcode:1993PhRvL..70..178L. doi:10.1103/PhysRevLett.70.178. PMID 10053722.
- ^ Fukui, Hiroshi; Hirao, Naohisa; Ohishi, Yasuo; Baron, Alfred Q R (10 March 2010). "Compressional behavior of solid NeHe2 up to 90 GPa". Journal of Physics: Condensed Matter. 22 (9): 095401. Bibcode:2010JPCM...22i5401F. doi:10.1088/0953-8984/22/9/095401. PMID 21389413. S2CID 41761505.
- ^ a b Teeratchanan, Pattanasak; Hermann, Andreas (21 October 2015). "Computational phase diagrams of noble gas hydrates under pressure" (PDF). The Journal of Chemical Physics. 143 (15): 154507. Bibcode:2015JChPh.143o4507T. doi:10.1063/1.4933371. hdl:20.500.11820/49320f15-083a-4b90-880b-6a670ad8c162. PMID 26493915.
- ^ a b c Kuhs, Werner F.; Hansen, Thomas C.; Falenty, Andrzej (29 May 2018). "Filling Ices with Helium and the Formation of Helium Clathrate Hydrate". The Journal of Physical Chemistry Letters. 9 (12): 3194–3198. doi:10.1021/acs.jpclett.8b01423. PMID 29809013. S2CID 46923138.
- ^ Zakharov, B. A.; Seryotkin, Y. V.; Tumanov, N. A.; Paliwoda, D.; Hanfland, M.; Kurnosov, A. V.; Boldyreva, E. V. (2016). "The role of fluids in high-pressure polymorphism of drugs: different behaviour of β-chlorpropamide in different inert gas and liquid media". RSC Advances. 6 (95): 92629–92637. Bibcode:2016RSCAd...692629Z. doi:10.1039/c6ra17750f.
- ^ Yagotintsev, K. A.; Strzhemechny, M. A.; Stetsenko, Yu. E.; Legchenkova, I. V.; Prokhvatilov, A. I. (May 2006). "Diffusion of He atoms in fullerite". Physica B: Condensed Matter. 381 (1–2): 224–232. Bibcode:2006PhyB..381..224Y. doi:10.1016/j.physb.2006.01.010.
- ^ Stetsenko, Yu. E.; Legchenkova, I. V.; Yagotintsev, K. A.; Prokhvatilov, A. I.; Strzhemechnyı̆, M. A. (May 2003). "Intercalation of C60 fullerite with helium and argon at normal temperature and pressure". Low Temperature Physics. 29 (5): 445–448. Bibcode:2003LTP....29..445S. doi:10.1063/1.1542509.
- ^ Grochala, Wojciech (2011-06-22). Khriachtchev, Leonid (ed.). Physics and Chemistry at Low Temperatures. Pan Stanford. p. 428. ISBN 9789814267519.
- ^ Morinaka, Yuta; Sato, Satoru; Wakamiya, Atsushi; Nikawa, Hidefumi; Mizorogi, Naomi; Tanabe, Fumiyuki; Murata, Michihisa; Komatsu, Koichi; Furukawa, Ko; Kato, Tatsuhisa; Nagase, Shigeru; Akasaka, Takeshi; Murata, Yasujiro (5 March 2013). "X-ray observation of a helium atom and placing a nitrogen atom inside He@C60 and He@C70". Nature Communications. 4 (1): 1554. Bibcode:2013NatCo...4.1554M. doi:10.1038/ncomms2574. PMID 23462997.

- ^ a b Onishi, Taku (19 May 2015). "A Molecular Orbital Analysis on Helium Dimer and Helium-Containing Materials". Journal of the Chinese Chemical Society. 63: 83–86. doi:10.1002/jccs.201500046.
- ^ Zou, Wenli; Liu, Yang; Liu, Wenjian; Wang, Ting; Boggs, James E. (14 January 2010). "He@Mo6Cl8F6: A Stable Complex of Helium". The Journal of Physical Chemistry A. 114 (1): 646–651. Bibcode:2010JPCA..114..646Z. doi:10.1021/jp908254r. PMID 19950905.
- ^ a b Efimov, V. B.; Mezhov-Deglin, L. P.; Dewhurst, C. D.; Lokhov, A. V.; Nesvizhevsky, V. V. (2015). "Neutron Scattering on Impurity Nanoclusters in Gel Samples". Advances in High Energy Physics. 2015: 1–4. doi:10.1155/2015/808212.
- ^ a b Kiselev, S. I.; Khmelenko, V. V.; Lee, D. M.; Kiryukhin, V.; Boltnev, R. E.; Gordon, E. B.; Keimer, B. (19 December 2001). "Structural studies of impurity-helium solids". Physical Review B. 65 (2): 024517. Bibcode:2001PhRvB..65b4517K. doi:10.1103/PhysRevB.65.024517.
- ^ a b c d e Khmelenko, V. V.; Kunttu, H.; Lee, D. M. (11 May 2007). "Recent Progress in Studies of Nanostructured Impurity–Helium Solids". Journal of Low Temperature Physics. 148 (1–2): 1–31. Bibcode:2007JLTP..148....1K. doi:10.1007/s10909-007-9353-6. S2CID 122589619.
- ^ a b Khmelenko, V. V.; Lee, D. M.; Vasiliev, S. (3 December 2010). "Matrix Isolation of H Atoms at Low Temperatures". Journal of Low Temperature Physics. 162 (3–4): 105–120. Bibcode:2011JLTP..162..105K. doi:10.1007/s10909-010-0302-4. S2CID 89615612.
- ^ a b c d e f Gordon, E.B.; Khmelenko, V.V.; Pelmenev, A.A.; Popov, E.A.; Pugachev, O.F. (March 1989). "Impurity-helium van der Waals crystals". Chemical Physics Letters. 155 (3): 301–304. Bibcode:1989CPL...155..301G. doi:10.1016/0009-2614(89)85329-1.
- ^ Boltnev, R. E. (2005). "Study of the stabilization and recombination of nitrogen atoms in impurity–helium condensates". Low Temperature Physics. 31 (7): 547–555. Bibcode:2005LTP....31..547B. doi:10.1063/1.2001631.
- ^ a b c Gordon, E.B.; Khmelenko, V.V.; Pelmenev, A.A.; Popov, E.A.; Pugachev, O.F.; Shestakov, A.F. (March 1993). "Metastable impurity-helium solid phase. Experimental and theoretical evidence". Chemical Physics. 170 (3): 411–426. Bibcode:1993CP....170..411G. doi:10.1016/0301-0104(93)85122-O.
- ^ a b c d Boltnev, R.E.; Gordon, E.B.; Khmelenko, V.V.; Krushinskaya, I.N.; Martynenko, M.V.; Pelmenev, A.A.; Popov, E.A.; Shestakov, A.F. (December 1994). "Luminescence of nitrogen and neon atoms isolated in solid helium". Chemical Physics. 189 (2): 367–382. Bibcode:1994CP....189..367B. doi:10.1016/0301-0104(94)00337-8.
- ^ Gordon, E. B.; Nishida, R.; Nomura, R.; Okuda, Y. (August 2007). "Filament formation by impurities embedding into superfluid helium". JETP Letters. 85 (11): 581–584. doi:10.1134/S0021364007110112. S2CID 120726845.
- ^ Boltnev, R. E.; Bernard, E. P.; Järvinen, J.; Krushinskaya, I. N.; Khmelenko, V. V.; Lee, D. M. (25 September 2009). "Stabilization of H and D atoms in Aggregates of Kr Nanoclusters Immersed in Superfluid Helium". Journal of Low Temperature Physics. 158 (3–4): 468–477. Bibcode:2010JLTP..158..468B. doi:10.1007/s10909-009-9961-4. S2CID 121373546.
- ^ Boltnev, R. E.; Khmelenko, V. V.; Lee, D. M. (2010). "Stabilization of H and D atoms in krypton–helium nanocondensates". Low Temperature Physics. 36 (5): 382. Bibcode:2010LTP....36..382B. doi:10.1063/1.3432245.
- ^ Boltnev, R. E.; Bernard, E. P.; Järvinen, J.; Khmelenko, V. V.; Lee, D. M. (14 May 2009). "Stabilization of hydrogen atoms in aggregates of krypton nanoclusters immersed in superfluid helium". Physical Review B. 79 (18): 180506. Bibcode:2009PhRvB..79r0506B. doi:10.1103/PhysRevB.79.180506.
- ^ Efimov, V.B.; Izotov, A.N.; Lokhov, A.V.; Mezhov-Deglin, L.P.; Nesvizhevsky, V.V.; Dewhurst, C.; Honecker, D. (19 April 2016). "SANS and X-Ray Scattering Study of Structure and Phase Transitions in Impurity-Helium Gel Samples and Fine Powders Created on Decay of the Gels" (PDF). Retrieved 14 July 2016.
- ^ Mezhov-Deglin, Leonid P.; Kokotin, Andrey M. (May 2003). "Water–helium condensate (watergel) in liquid helium". Physica B: Condensed Matter. 329–333: 331–332. Bibcode:2003PhyB..329..331M. CiteSeerX 10.1.1.489.467. doi:10.1016/S0921-4526(02)02074-4.
- ^ Efimov, V. B.; Lokhov, A. V.; Mezhov-Deglin, L. P.; Dewhurst, C.; Nesvizhevsky, V. V.; Kolmakov, G. V. (26 March 2014). "Nanocluster magnetic gel in superfluid He-II". JETP Letters. 99 (1): 32–36. Bibcode:2014JETPL..99...32E. doi:10.1134/S0021364014010044. S2CID 120144532.
- ^ a b Moroshkin, P.; Hofer, A.; Ulzega, S.; Weis, A. (23 September 2007). "Impurity-stabilized solid 4He below the solidification pressure of pure helium" (PDF). Nature Physics. 3 (11): 786–789. Bibcode:2007NatPh...3..786M. doi:10.1038/nphys727.
- ^ Batulin, R.; Moroshkin, P.; Tayurskii, D. A.; Kono, K. (January 2018). "Spectroscopy of Ba+ ions in liquid 4He". AIP Advances. 8 (1): 015328. Bibcode:2018AIPA....8a5328B. doi:10.1063/1.5011447.
- ^ Moroshkin, P.; Hofer, A.; Weis, A. (November 2008). "Atomic and molecular defects in solid 4He" (PDF). Physics Reports. 469 (1): 1–57. Bibcode:2008PhR...469....1M. doi:10.1016/j.physrep.2008.06.004.
- ^ a b c Blackburn, R. (19 July 2013). "Inert Gases in Metals". Metallurgical Reviews. 11 (1): 159–176. doi:10.1179/mtlr.1966.11.1.159.
- ^ Adams, J. B.; Wolfer, W. G.; Foiles, S. M.; Rohlfing, C. M.; van Siclen, C. D. (16 September 1990). "Theoretical Studies of Helium in Metals". In Donnelly, S.E.; Evans, J.H. (eds.). Fundamental Aspects of Inert Gases in Solids. Springer. pp. 3–16. ISBN 9781489936806.
- ^ Moroshkin, P.; Lebedev, V.; Grobety, B.; Neururer, C.; Gordon, E. B.; Weis, A. (1 May 2010). "Nanowire formation by gold nano-fragment coalescence on quantized vortices in He II" (PDF). EPL. 90 (3): 34002. Bibcode:2010EL.....9034002M. doi:10.1209/0295-5075/90/34002. S2CID 55800041.
- ^ Gordon, E B; Karabulin, A V; Matyushenko, V I; Sizov, V D; Khodos, I I (1 September 2015). "Production of ultrathin nanowires from refractory metals (Nb, Re, W, Mo) by laser ablation in superfluid helium". Laser Physics Letters. 12 (9): 096002. Bibcode:2015LaPhL..12i6002G. doi:10.1088/1612-2011/12/9/096002. S2CID 124394791.
- ^ Gordon, Eugene B.; Karabulin, Alexander Vladimirovich; Kulish, Mikhail I.; Matyushenko, Vladimir Igorevich; Stepanov, Maxim E. (17 November 2017). "Coagulation of Metals in Superfluid and Normal Liquid Helium". The Journal of Physical Chemistry A. 121 (48): 9185–9190. Bibcode:2017JPCA..121.9185G. doi:10.1021/acs.jpca.7b08645. PMID 29148776.
- ^ Gordon, E. B.; Karabulin, A. V.; Matyushenko, V. I.; Sizov, V. D.; Khodos, I. I. (2012). "The electrical conductivity of bundles of superconducting nanowires produced by laser ablation of metals in superfluid helium". Applied Physics Letters. 101 (5): 052605. Bibcode:2012ApPhL.101e2605G. doi:10.1063/1.4742330.
- ^ Gordon, E. B.; Karabulin, A. V.; Matyushenko, V. I.; Sizov, V. D.; Khodos, I. I. (14 July 2011). "Structure of metallic nanowires and nanoclusters formed in superfluid helium". Journal of Experimental and Theoretical Physics. 112 (6): 1061–1070. Bibcode:2011JETP..112.1061G. doi:10.1134/S1063776111040182. S2CID 119874763.
- ^ Gordon, Eugene B.; Karabulin, Alexander V.; Matyushenko, Vladimir I.; Sizov, Vyacheslav D.; Khodos, Igor I. (5 January 2013). "The Nanostructures Produced by Laser Ablation of Metals in Superfluid Helium". Journal of Low Temperature Physics. 172 (1–2): 94–112. Bibcode:2013JLTP..172...94G. doi:10.1007/s10909-012-0849-3. S2CID 119677151.
- ^ Elliott, P. L.; Pakes, C. I.; Skrbek, L.; Vinen, W. F. (1 January 2000). "Capillary-wave crystallography: Crystallization of two-dimensional sheets of He+ ions". Physical Review B. 61 (2): 1396–1409. Bibcode:2000PhRvB..61.1396E. doi:10.1103/PhysRevB.61.1396.
- ^ Friedrich, Bretislav (8 April 2013). "A Fragile Union Between Li and He Atoms". Physics. 6: 42. Bibcode:2013PhyOJ...6...42F. doi:10.1103/Physics.6.42. hdl:11858/00-001M-0000-000E-F3C4-C.
- ^ Brahms, N.; Tscherbul, T. V.; Zhang, P.; Kłos, J.; Sadeghpour, H. R.; Dalgarno, A.; Doyle, J. M.; Walker, T. G. (2010). "Formation of van der Waals molecules in buffer gas cooled magnetic traps". Physical Review Letters. 105 (3): 033001. arXiv:1003.0948. Bibcode:2010PhRvL.105c3001B. doi:10.1103/PhysRevLett.105.033001. PMID 20867761. S2CID 12125566.
- ^ Bergeat, Astrid; Onvlee, Jolijn; Naulin, Christian; van der Avoird, Ad; Costes, Michel (24 March 2015). "Quantum dynamical resonances in low-energy CO(j = 0) + He inelastic collisions". Nature Chemistry. 7 (4): 349–353. Bibcode:2015NatCh...7..349B. doi:10.1038/nchem.2204. PMID 25803474.
- ^ Cappelletti, David; Bartocci, Alessio; Grandinetti, Felice; Falcinelli, Stefano; Belpassi, Leonardo; Tarantelli, Francesco; Pirani, Fernando (13 April 2015). "Experimental Evidence of Chemical Components in the Bonding of Helium and Neon with Neutral Molecules". Chemistry: A European Journal. 21 (16): 6234–6240. doi:10.1002/chem.201406103. PMID 25755007.
- ^ Smalley, R. E. (1976). "The fluorescence excitation spectrum of the HeI2 van der Waals complex". The Journal of Chemical Physics. 64 (8): 3266–3276. Bibcode:1976JChPh..64.3266S. doi:10.1063/1.432667.
- ^ Higgins, J. P.; Reho, J.; Stienkemeier, F.; Ernst, W. E.; Lehmann, K. K.; Scoles, G. (2001). "Spectroscopy in, on, and off a Beam of Superfluid Helium Nanodroplets". Atomic and Molecular Beams. pp. 723–754. doi:10.1007/978-3-642-56800-8_51. ISBN 978-3-642-63150-4.
- ^ Yang, Tiangang; Yang, Xueming (7 May 2020). "Quantum resonances near absolute zero". Science. 368 (6491): 582–583. Bibcode:2020Sci...368..582Y. doi:10.1126/science.abb8020. PMID 32381705. S2CID 218552023.
- ^ Scheidemann, A.; Schilling, B.; Toennies, J. Peter (March 1993). "Anomalies in the reactions of He+ with SF6 embedded in large helium-4 clusters". The Journal of Physical Chemistry. 97 (10): 2128–2138. doi:10.1021/j100112a012.
- ^ a b c d e f g h i j k l m n o p Grandinetti, Felice (October 2004). "Helium chemistry: a survey of the role of the ionic species". International Journal of Mass Spectrometry. 237 (2–3): 243–267. Bibcode:2004IJMSp.237..243G. doi:10.1016/j.ijms.2004.07.012.
- ^ a b c d e f g h i j k l m n o p Gao, Kunqi (2015). "Theoretical investigation of HNgNH3+ ions (Ng = He, Ne, Ar, Kr, and Xe)". Journal of Chemical Physics. 142 (14): 144301. Bibcode:2015JChPh.142n4301G. doi:10.1063/1.4916648. PMID 25877572.
- ^ Patterson, P. L. (1968). "Evidence of the Existence of an He3+ Ion". Journal of Chemical Physics. 48 (8): 3625. Bibcode:1968JChPh..48.3625P. doi:10.1063/1.1669660.
- ^ Bartl, Peter; Leidlmair, Christian; Denifl, Stephan; Scheier, Paul; Echt, Olof (14 January 2013). "Cationic Complexes of Hydrogen with Helium". ChemPhysChem. 14 (1): 227–232. doi:10.1002/cphc.201200664. PMC 3555426. PMID 23090688.
- ^ a b c Kim, Jeong Hyun; Peterka, Darcy S.; Wang, Chia C.; Neumark, Daniel M. (2006). "Photoionization of helium nanodroplets doped with rare gas atoms". The Journal of Chemical Physics. 124 (21): 214301. Bibcode:2006JChPh.124u4301K. doi:10.1063/1.2202313. PMID 16774401.
- ^ Callicoatt, Berton E.; Förde, Kirk; Ruchti, Thomas; Jung, Lilian; Janda, Kenneth C.; Halberstadt, Nadine (1998). "Capture and ionization of argon within liquid helium droplets". The Journal of Chemical Physics. 108 (22): 9371. Bibcode:1998JChPh.108.9371C. doi:10.1063/1.476389.
- ^ a b c Tsong, T. T. (1983). "Field induced and surface catalyzed formation of novel ions: A pulsed-laser time-of-flight atom-probe study". The Journal of Chemical Physics. 78 (7): 4763–4775. Bibcode:1983JChPh..78.4763T. doi:10.1063/1.445276.
- ^ a b AnderLan, Lukas; Bartl, Peter; Leidlmair, Christian; Jochum, Roland; Denifl, Stephan; Echt, Olof; Scheier, Paul (2 April 2012). "Solvation of Na+, K+, and Their Dimers in Helium". Chemistry: A European Journal. 18 (14): 4411–4418. doi:10.1002/chem.201103432. PMC 3350777. PMID 22374575.
- ^ Lammertsma, Koop; von Rague Schleyer, Paul; Schwarz, Helmut (October 1989). "Organic Dications: Gas Phase Experiments and Theory in Concert". Angewandte Chemie International Edition in English. 28 (10): 1321–1341. doi:10.1002/anie.198913211.
- ^ Olah, George A.; Klumpp, Douglas A. (2008). Superelectrophiles and their Chemistry. John Wiley. ISBN 9780470049617.
- ^ Liu, J.; Tsong, T. T. (November 1988). "High Resolution Ion Kinetic Energ Analysis of Field Emitted Ions". Le Journal de Physique Colloques. 49 (C6): C6–61–C6–66. doi:10.1051/jphyscol:1988611.
- ^ Datz, Sheldon (22 Oct 2013). Condensed Matter: Applied Atomic Collision Physics, Vol. 4. Academic Press. p. 391. ISBN 9781483218694.
- ^ Jašík, Juraj; Žabka, Ján; Roithová, Jana; Gerlich, Dieter (November 2013). "Infrared spectroscopy of trapped molecular dications below 4K". International Journal of Mass Spectrometry. 354–355: 204–210. Bibcode:2013IJMSp.354..204J. doi:10.1016/j.ijms.2013.06.007.
- ^ a b c d Nizkorodov, S. A.; Maier, J. P.; Bieske, E. J. (1995). "The infrared spectrum of He–HCO+". The Journal of Chemical Physics. 103 (4): 1297–1302. Bibcode:1995JChPh.103.1297N. doi:10.1063/1.469806.
- ^ Campbell, E. K.; Holz, M.; Gerlich, D.; Maier, J. P. (15 July 2015). "Laboratory confirmation of C60+ as the carrier of two diffuse interstellar bands". Nature. 523 (7560): 322–323. Bibcode:2015Natur.523..322C. doi:10.1038/nature14566. PMID 26178962. S2CID 205244293.
- ^ Frenking, Gernot; Koch, Wolfram; Reichel, Felix; Cremer, Dieter (May 1990). "Light noble gas chemistry: structures, stabilities, and bonding of helium, neon, and argon compounds". Journal of the American Chemical Society. 112 (11): 4240–4256. doi:10.1021/ja00167a020.
- ^ Zhdankin, V. V. (November 1993). "Organic chemistry of noble gases". Russian Chemical Bulletin. 42 (11): 1763–1771. doi:10.1007/BF00698985. S2CID 97379406.
- ^ Nizkorodov, S. A.; Maier, J. P.; Bieske, E. J. (1995). "The infrared spectrum of the N2H+–He ion-neutral complex" (PDF). The Journal of Chemical Physics. 102 (13): 5570. Bibcode:1995JChPh.102.5570N. doi:10.1063/1.469286.
- ^ a b Meuwly, M.; Nizkorodov, S. A.; Maier, J. P.; Bieske, E. J. (1996). "Mid-infrared spectra of He–HN+2 and He2–HN+2". The Journal of Chemical Physics. 104 (11): 3876–3885. Bibcode:1996JChPh.104.3876M. doi:10.1063/1.471244.
- ^ Albertini, Simon; Bergmeister, Stefan; Laimer, Felix; Martini, Paul; Gruber, Elisabeth; Zappa, Fabio; Ončák, Milan; Scheier, Paul; Echt, Olof (2021-04-22). "SF6+: Stabilizing Transient Ions in Helium Nanodroplets". The Journal of Physical Chemistry Letters. 12 (17): 4112–4117. doi:10.1021/acs.jpclett.1c01024. PMC 8154854. PMID 33886323.
- ^ Moroshkin, P.; Kono, K. (29 April 2016). "Bound-bound transitions in the emission spectra of Ba+–He excimer". Physical Review A. 93 (5): 052510. arXiv:1604.08700. Bibcode:2016PhRvA..93e2510M. doi:10.1103/PhysRevA.93.052510. S2CID 119246040.
- ^ Liu, Hanyu; Yao, Yansun; Klug, Dennis D. (7 January 2015). "Stable structures of He and H2O at high pressure". Physical Review B. 91 (1): 014102. Bibcode:2015PhRvB..91a4102L. doi:10.1103/PhysRevB.91.014102. S2CID 124928082.
- ^ a b H. Damianovich, Anales del Instituto de Investigaciones Científicas y Technológicas, 1932, 1, 30.; H. Damianovich, Anales del Instituto de Investigaciones Científicas y Technológicas, 1934, 3/4, 20.; H. Damianovich C Christer, Revista Brasilera de Chimica, São Paulo, 1938 6 72; H. Damianovich, Anales de la Sociedad Científica Argentina, 1934, 118, 227.; H. Damianovich, Bulletin de la Société Chimique de France, 1938, 5, 1085.; H. Damianovich Anales de la Sociedad Española de Física y Química 1928. 26. 365; H. Damianovich. 7thProc.Am.Sci.Congr., Phys.Chem Chem.Sci.1940, 137;not consulted
- ^ Madhu Chatwal, ed. (2008). Advanced Inorganic Chemistry Vol-1. Krishna Prakashan Media. p. 834. ISBN 978-81-87224-03-7.
- ^ Ruffini, Remo (1975). "The Physics of Gravitationally Collapsed Objects". Neutron Stars, Black Holes and Binary X-Ray Sources. Astrophysics and Space Science Library. Vol. 48. pp. 59–118. Bibcode:1975ASSL...48..119G. doi:10.1007/978-94-010-1767-1_5. ISBN 978-90-277-0542-6.
- ^ Dyson, Freeman J (March 1971). "Chemical binding in classical Coulomb lattices". Annals of Physics. 63 (1): 1–11. Bibcode:1971AnPhy..63....1D. doi:10.1016/0003-4916(71)90294-6.
- ^ Monserrat, Bartomeu; Martinez-Canales, Miguel; Needs, Richard; Pickard, Chris (July 2018). "Helium–Iron Compounds at Terapascal Pressures". Physical Review Letters. 121 (1): 015301. arXiv:1806.03017. Bibcode:2018PhRvL.121a5301M. doi:10.1103/PhysRevLett.121.015301. PMID 30028166. S2CID 51702435.
- ^ Bradley, David (6 February 2017). "Pressing helium discovery as gas reacted with sodium". Chemistry World.
- ^ Liu, Zhen; Botana, Jorge; Hermann, Andreas; Valdez, Steven; Zurek, Eva; Yan, Dadong; Lin, Hai-qing; Miao, Mao-sheng (5 March 2018). "Reactivity of He with ionic compounds under high pressure". Nature Communications. 9 (1): 951. Bibcode:2018NatCo...9..951L. doi:10.1038/s41467-018-03284-y. PMC 5838161. PMID 29507302.
- ^ Li, Tianshu; Xu, Enshi; Bi, Yuanfei (22 March 2018). "Formation of inclusion type silicon phases induced by inert gases". Communications Chemistry. 1 (1): 15. doi:10.1038/s42004-018-0013-3.
- ^ a b c Motegi, Haruki; Kakizaki, Akira; Takayanagi, Toshiyuki; Taketsugu, Yuriko; Taketsugu, Tetsuya; Shiga, Motoyuki (December 2008). "Path-integral molecular dynamics simulations of BeO embedded in helium clusters: Formation of the stable HeBeO complex". Chemical Physics. 354 (1–3): 38–43. Bibcode:2008CP....354...38M. doi:10.1016/j.chemphys.2008.09.001.
- ^ a b c Kobayashi, Takanori; Kohno, Yuji; Takayanagi, Toshiyuki; Seki, Kanekazu; Ueda, Kazuyoshi (July 2012). "Rare gas bond property of Rg–Be2O2 and Rg–Be2O2–Rg (Rg=He, Ne, Ar, Kr and Xe) as a comparison with Rg–BeO". Computational and Theoretical Chemistry. 991: 48–55. doi:10.1016/j.comptc.2012.03.020.
- ^ Borocci, S; Bronzolino, N; Grandinetti, F (23 June 2006). "Neutral helium compounds: theoretical evidence for a large class of polynuclear complexes". Chemistry: A European Journal. 12 (19): 5033–42. doi:10.1002/chem.200600219. PMID 16642536.
- ^ Saha, Ranajit; Pan, Sudip; Chattaraj, Pratim Kumar (19 April 2017). "NgMCp+: Noble Gas Bound Half-Sandwich Complexes (Ng = He–Rn, M = Be–Ba, Cp = η5-C5H5)". The Journal of Physical Chemistry A. 121 (18): 3526–3539. Bibcode:2017JPCA..121.3526S. doi:10.1021/acs.jpca.7b00389. PMID 28423279.
- ^ a b c d Zou, Wenli; Liu, Yang; Boggs, James E. (November 2009). "Theoretical study of RgMF (Rg=He, Ne; M=Cu, Ag, Au): Bonded structures of helium". Chemical Physics Letters. 482 (4–6): 207–210. Bibcode:2009CPL...482..207Z. doi:10.1016/j.cplett.2009.10.010.
- ^ Chaban, Galina M.; Lundell, Jan; Gerber, R. Benny (2001). "Lifetime and decomposition pathways of a chemically bound helium compound". The Journal of Chemical Physics. 115 (16): 7341. Bibcode:2001JChPh.115.7341C. doi:10.1063/1.1412467.
- ^ Bihary, Z.; Chaban, G. M.; Gerber, R. B. (2002). "Stability of a chemically bound helium compound in high-pressure solid helium". The Journal of Chemical Physics. 117 (11): 5105. Bibcode:2002JChPh.117.5105B. doi:10.1063/1.1506150.
- ^ a b c d e f g h i j k l m n o p q r s t u v w Brahms, Nathan; Tscherbul, Timur V.; Zhang, Peng; Kłos, Jacek; Forrey, Robert C.; Au, Yat Shan; Sadeghpour, H. R.; Dalgarno, A.; Doyle, John M.; Walker, Thad G. (2011). "Formation and dynamics of van der Waals molecules in buffer-gas traps". Physical Chemistry Chemical Physics. 13 (42): 19125–41. arXiv:1104.4973. Bibcode:2011PCCP...1319125B. doi:10.1039/C1CP21317B. PMID 21808786. S2CID 2361186.
- ^ Valdes, Alvaro; Prosmiti, Rita (3 December 2015). "Vibrational Calculations of Higher-Order Weakly Bound Complexes: the He3,4I2 Cases". The Journal of Physical Chemistry A. 119 (51): 12736–12741. Bibcode:2015JPCA..11912736V. doi:10.1021/acs.jpca.5b10398. hdl:10261/135396. PMID 26634405.
- ^ Grochala, Wojciech (2012). "A metastable He–O bond inside a ferroelectric molecular cavity: (HeO)(LiF)2". Physical Chemistry Chemical Physics. 14 (43): 14860–8. Bibcode:2012PCCP...1414860G. doi:10.1039/C2CP42321A. PMID 23037895.
- ^ Pan, Sudip; Saha, Ranajit; Chattaraj, Pratim K. (2015). "On the stability of noble gas bound 1-tris(pyrazolyl)borate beryllium and magnesium complexes". New J. Chem. 39 (9): 6778–6786. doi:10.1039/C5NJ00983A.
- ^ Grochala, W. (2009). "On Chemical Bonding Between Helium and Oxygen". Polish Journal of Chemistry. 83 (1): 87–122.
- ^ Kolganova, E. A. (24 January 2017). "Weakly Bound LiHe2 Molecules". Few-Body Systems. 58 (2): 57. arXiv:1612.03820. Bibcode:2017FBS....58...57K. doi:10.1007/s00601-017-1222-5. S2CID 100472055.
- ^ Wesendrup, Ralf; Pernpointner, Markus; Schwerdtfeger, Peter (November 1999). "Coulomb-stable triply charged diatomic: HeY3+". Physical Review A. 60 (5): R3347–R3349. Bibcode:1999PhRvA..60.3347W. doi:10.1103/PhysRevA.60.R3347.
- ^ Di Rienzi, Joseph; Drachman, Richard (February 2007). "Nonradiative formation of the positron-helium triplet bound state". Physical Review A. 75 (2): 024501. Bibcode:2007PhRvA..75b4501D. doi:10.1103/PhysRevA.75.024501.
- ^ Li, Tsung-Hui; Mou, Chun-Hao; Chen, Hui-Ru; Hu, Wei-Ping (June 2005). "Theoretical Prediction of Noble Gas Containing Anions FNgO−(Ng = He, Ar, and Kr)". Journal of the American Chemical Society. 127 (25): 9241–9245. doi:10.1021/ja051276f. PMID 15969603.
- ^ Jayasekharan, T.; Ghanty, T. K. (2008). "Theoretical prediction of HRgCO+ ion (Rg=He, Ne, Ar, Kr, and Xe)". The Journal of Chemical Physics. 129 (18): 184302. Bibcode:2008JChPh.129r4302J. doi:10.1063/1.3008057. PMID 19045398.
- ^ Borocci, Stefano; Bronzolino, Nicoletta; Grandinetti, Felice (June 2008). "Noble gas–sulfur anions: A theoretical investigation of FNgS− (Ng=He, Ar, Kr, Xe)". Chemical Physics Letters. 458 (1–3): 48–53. Bibcode:2008CPL...458...48B. doi:10.1016/j.cplett.2008.04.098.
- ^ Jayasekharan, T.; Ghanty, T. K. (2012). "Theoretical investigation of rare gas hydride cations: HRgN2+ (Rg=He, Ar, Kr, and Xe)". The Journal of Chemical Physics. 136 (16): 164312. Bibcode:2012JChPh.136p4312J. doi:10.1063/1.4704819. PMID 22559487.
- ^ Antoniotti, Paola; Benzi, Paola; Bottizzo, Elena; Operti, Lorenza; Rabezzana, Roberto; Borocci, Stefano; Giordani, Maria; Grandinetti, Felice (August 2013). "(HNg+)(OH2) complexes (Ng=He–Xe): An ab initio and DFT theoretical investigation". Computational and Theoretical Chemistry. 1017: 117–125. doi:10.1016/j.comptc.2013.05.015.
- ^ a b Page, Alister J.; von Nagy-Felsobuki, Ellak I. (November 2008). "Structural and energetic trends in Group-I and II hydrohelide cations". Chemical Physics Letters. 465 (1–3): 10–14. Bibcode:2008CPL...465...10P. doi:10.1016/j.cplett.2008.08.106.
- ^ Borocci, Stefano; Bronzolino, Nicoletta; Grandinetti, Felice (November 2004). "OBHe+: a remarkably stable singly charged cation containing helium". Chemical Physics Letters. 398 (4–6): 357–360. Bibcode:2004CPL...398..357B. doi:10.1016/j.cplett.2004.09.096.
- ^ Wheeler, Henry P.; Swenarton, Louise B. (1952). "Helium: Bibliography of Technical and Scientific Literature from Its Discovery (1868) to January 1, 1947". United States. Bureau of Mines. pp. 25–27. Retrieved 9 February 2017.
- ^ Troost, L.; Ouvrard, L. (1895). "Sur la combinaison du magnésium avec l'argon et avec l'hélium". Comptes Rendus de l'Académie des Sciences (in French). 121: 394–395. Retrieved 16 May 2016.
- ^ Cooke, W Ternant (8 February 1906). "Experiments on the Chemical Behaviour of Argon and Helium". Proceedings of the Royal Society of London. Series A. 77 (515): 148–. Bibcode:1906RSPSA..77..148C. doi:10.1098/rspa.1906.0014.
- ^ Heller, Ralph (1941). "Theory of Some van der Waals Molecules". The Journal of Chemical Physics. 9 (2): 154–163. Bibcode:1941JChPh...9..154H. doi:10.1063/1.1750868.paywalled;
- ^ Manley, J. J. (7 March 1925). "Mercury Helide". Nature. 115 (2888): 337. Bibcode:1925Natur.115..337M. doi:10.1038/115337d0. S2CID 4122049.
- ^ Manley, J. J. (20 June 1925). "Mercury Helide: a Correction". Nature. 115 (2903): 947. Bibcode:1925Natur.115..947M. doi:10.1038/115947d0. S2CID 4122263.
- ^ Manley, J. J. (13 December 1924). "Mercury and Helium". Nature. 114 (2876): 861. Bibcode:1924Natur.114Q.861M. doi:10.1038/114861b0. S2CID 41395470.
- ^ Manley, J. J. (1931). "The Discovery of Mercury Helide". Proceedings of the Bournemouth Natural Science Society. XXIII: 61–63.
- ^ Vernengo, Marcelo (July 2001). "La química en la Argentina de entreguerras" (PDF). Saber y Tiempo. 3 (12): 159. Retrieved 16 May 2016.
- ^ a b Waller, J. G. (7 May 1960). "New Clathrate Compounds of the Inert Gases". Nature. 186 (4723): 429–431. Bibcode:1960Natur.186..429W. doi:10.1038/186429a0. S2CID 4299293.
- ^ Boomer, E. H. (1 September 1925). "Experiments on the Chemical Activity of Helium". Proceedings of the Royal Society of London. Series A. 109 (749): 198–205. Bibcode:1925RSPSA.109..198B. doi:10.1098/rspa.1925.0118. JSTOR 94507.
- ^ a b Boomer, E. H. (3 January 1925). "Chemical Combination of Helium". Nature. 115 (2879): 16. Bibcode:1925Natur.115Q..16B. doi:10.1038/115016a0. S2CID 4020517.
- ^ Darpan, Pratiyogita (May 1999). Competition Science Vision.
- ^ Raj, Gurdeep. Advanced Inorganic Chemistry Vol-1. Krishna Prakashan Media. ISBN 9788187224037.
- ^ "Helium". Van Nostrand's Scientific Encyclopedia. John Wiley & Sons. 2005. doi:10.1002/0471743984.vse3860. ISBN 978-0471743989.
- ^ Krefft, H.; Rompe, R. (14 August 2013). "Über das Auftreten von Metall-Edelgasbanden in der positiven Säule elektrischer Entladungen". Zeitschrift für Physik (in German). 73 (9–10): 681–690. Bibcode:1932ZPhy...73..681K. doi:10.1007/BF01342016. S2CID 124198549.
Further reading
[edit]- Bhattacharya, Sayak (January 2016). "Quantum dynamical studies of the He + HeH+ reaction using multi-configuration time-dependent Hartree approach". Computational and Theoretical Chemistry. 1076: 81–85. doi:10.1016/j.comptc.2015.12.018.
External links
[edit] Media related to Helium compounds at Wikimedia Commons
Media related to Helium compounds at Wikimedia Commons
