GNLY
This article includes a list of general references, but it lacks sufficient corresponding inline citations. (June 2019) |
| GNLY | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | GNLY, 519, D2S69E, LAG-2, LAG2, NKG5, TLA519, granulysin | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 188855; HomoloGene: 136805; GeneCards: GNLY; OMA:GNLY - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Granulysin (GNLY) is a protein expressed in most mammals which functions as an antimicrobial peptide released by killer lymphocytes in cytotoxic granules.[3][4] It is a pore-forming peptide, as it can puncture a microbial cell wall, allowing for other death-inducing enzymes to enter the microbe and cause microptosis.[3] GNLY is inhibited by cholesterol, and is most effective in helping to kill cholesterol-deficient microbes.[5]
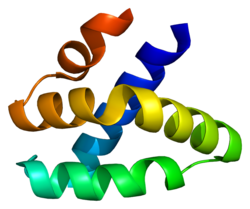
It is part of the saponin-like protein family, and its gene is found on the 2nd chromosome in humans.[3] It is distinguished by its 5 α-helical structure. Its expression is restricted to cytotoxic immune cells such as cytotoxic T cells, NK cells, NKT cells and γδ T cells.[4][3] Orthologs of this protein are found in most mammal species, such as in cows and pigs, however not in rodents.[5][3][4]
Granulysin is also an active player in many diseases, including leprosy and toxic epidermal necrolysis.[3]
Structure
[edit]Granulysin has a five alpha-helix structure, and is part of the saposin-like protein family.[5] It is expressed in 2 forms: a 15kDa precursor protein, the translation product, and a 9kDa cytotoxic protein, which is formed after cleavage of the amino and carbonyl ends of the 15kDa protein.[5][4][3]
The 15 kDa form consists of 145 amino acids, and is an inactive protein.[3] It exists in its own granule after translation, and release of the protein is triggered by Protein Kinase C (PKC).[5] Its C- and N-Termini function to properly direct the molecule to cytotoxic granules, and are subsequently cleaved once this has been achieved to prevent autolysis.[5] 15 kDa plays other roles in immunological processes, such as in antigen-presenting cell maturation and in immune cell migration.[5]
The 9 kDa form consists of 74 amino acids, and has a cytotoxic function. This molecule is found in cytotoxic granules, along with other cytotoxic molecules, such as granzymes and perforin.[5][4][3] The molecule's positive charge allows for binding to phospholipids and cardiolipin, both of which can be found as epitopes on the surfaces of pathogens, and its 2nd and 3rd helices are principle players in lysing foreign or infected cells.[5][3]
Gene expression
[edit]GNLY gene is located on human chromosome 2 and has 5 exons, which code for a 15 kDa protein.[3] The path to transcription has not been elucidated: transcription factors, promoter regions, and pathogen-associated molecular patterns, which likely induce the signaling pathway necessary for the eventual translation of this protein, are unknown.[3] Granulysin is expressed in killer cells, such as cytotoxic T cells and Natural Killer (NK) cells, which hold the cytotoxic granules this protein is contained in.[3][4] These cells can be found mainly in the epidermis to protect against infection spreading through the skin.[3] In addition, high expression of Granulysin can be found in the placenta to protect fetal epithelial cells.[3]
Function
[edit]15 kDa GNLY
[edit]The 15 kDa GNLY was originally thought to function exclusively as an inactive precursor of antimicrobial 9 kDa GNLY, however this hypothesis has been recently challenged.[5] 15 kDa has been shown to be located in its own granules and its release is governed by PKC, unlike the 9 kDa GNLY, which is released from its granules via Ca2+.[4] The 15 kDa also functions as an alarmin, molecules capable of starting an inflammatory response.[5] More precisely, 15 kDa GNLY is capable of initiating differentiation of monocytes into dendritic cells. The 15 kDa form is also able to act as a chemoattractant for different cells, such as NK cells, cytotoxic T cells, helper T cells, and in higher concentrations, immature dendritic cells.[5]
9 kDa GNLY
[edit]The 9 kDa form functions as a pore-forming protein, as it is able to permeabilize cell membranes.[3][5][4]The 9kDa form can cytolyze fungi, yeast, parasites, gram negative, and gram positive bacteria.[6] This protein is also far more effective in targeting bacterial membranes than mammalian membranes, though it can target many different cell types, such as those from fungi and parasites.[4] The 9 kDa form is also inhibited by cholesterol which is present in usually present in mammalian cells, but not in most pathogen cells.[3][4] This all makes GNLY 1000 times less effective in pore formation in human cells than in microbe cells. However, the precise mechanisms of pore formation is not yet fully understood.[5][4]
Although GNLY is able to kill pathogens by itself, usually, it cooperates with other proteins from cytotoxic granules, most notably with granzymes.[3] When a cytotoxic cell discovers any infected cell the content of the cytotoxic granules is released by receptor-mediated exocytosis.[3][4] Perforin, unlike GNLY, binds preferably to cholesterol rich membranes and permeabilizes the infected cell which allows the entry of GNLY and granzymes.[3] GNLY then creates pores in pathogen membranes so granzymes can move into the pathogen where it can cause microptosis.[4][3][5]
Granzymes usually cause apoptosis of the infected cell through initiation of the caspase cascade.[4] However, apoptosis can also be initiated by GNLY, due to the presence of cardiolipin in mitochondrial membranes which allows GNLY to create pores in the membrane and causing a release of molecules like cytochrome c, which also leads to apoptosis.[4][3]
Evolution
[edit]GNLY orthologues have been identified in multiple species including pigs, chicken, and cattle. Out of these species (human included) only in cattle 4 functional GNLY where characterized.[7] Generally, such gene duplication can lead to functional specification which seems to be the case of bovine GNLYs because of two reasons. First, the 4 genes are differentially expressed in different tissues.[8] Second, some common cattle pathogens like Histophilus somni and Mannheimia haemolytica have significantly different sensitivity to each of the 4 bovine GNLY.[9]
Clinical significance
[edit]Granulysin plays a role in a myriad of diseases, where it can be a positive or negative influence on the immune response. In Leprosy, for example, Granulysin acts to prevent further infection, and infected individuals often have higher expression of killer cells expressing Granulysin.[3] However, in diseases in which Granulysin is expressed in high concentrations individuals can have debilitating or life-threatening symptoms, most notably in autoimmune diseases where cells can be lysed by killer cells.[3]
Toxic epidermal necrolysis
[edit]Granulysin plays a large role in Toxic Epidermal Necrolysis (TEN), a disease in which patients suffer from severe blistering, destruction of mucus tissues, fluid loss, and inflamed skin, caused by an immune response to drugs.[10] A drug will often bind to the major histocompatibility complex type I (MHC-I) and cytotoxic T cell receptors, resulting in a cytotoxic immune response.[10] Granulysin has been determined to be the principal player in cell death in this disease. Individuals suffering from TEN were found to have high concentrations of Granulysin in their blister fluid.[10]
Cancer
[edit]Granulysin has also been shown to slow the progression of cancers and destroy transformed cells through apoptosis.[5] Patients with high levels of Granulysin in blood serum are better able to fight off metastasis, and generally progression of cancer stages is slow.[5] There is considerable evidence that the 9 kDa form is itself able to destroy tumor cells, however exactly how it does this has not been determined.[5] One mechanism of cell destruction is through initiating calcium increase, which harms the mitochondria and increases the level of cytochrome b, and eventually causing apoptosis.[5]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000115523 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x Dotiwala F, Lieberman J (October 2019). "Granulysin: killer lymphocyte safeguard against microbes". Current Opinion in Immunology. 60: 19–29. doi:10.1016/j.coi.2019.04.013. PMC 6800608. PMID 31112765.
- ^ a b c d e f g h i j k l m n o Liu X, Lieberman J (April 2020). "Knocking 'em Dead: Pore-Forming Proteins in Immune Defense". Annual Review of Immunology. 38 (1): 455–485. doi:10.1146/annurev-immunol-111319-023800. PMC 7260445. PMID 32004099.
- ^ a b c d e f g h i j k l m n o p q r s Sparrow E, Bodman-Smith MD (January 2020). "Granulysin: The attractive side of a natural born killer" (PDF). Immunology Letters. 217: 126–132. doi:10.1016/j.imlet.2019.11.005. PMID 31726187. S2CID 208036617.
- ^ Krensky AM, Clayberger C (March 2009). "Biology and clinical relevance of granulysin". Tissue Antigens. 73 (3): 193–198. doi:10.1111/j.1399-0039.2008.01218.x. PMC 2679253. PMID 19254247.
- ^ Endsley JJ, Furrer JL, Endsley MA, McIntosh MA, Maue AC, Waters WR, et al. (August 2004). "Characterization of bovine homologues of granulysin and NK-lysin". Journal of Immunology. 173 (4): 2607–2614. doi:10.4049/jimmunol.173.4.2607. PMID 15294977. S2CID 22595074.
- ^ Chen J, Huddleston J, Buckley RM, Malig M, Lawhon SD, Skow LC, et al. (December 2015). "Bovine NK-lysin: Copy number variation and functional diversification". Proceedings of the National Academy of Sciences of the United States of America. 112 (52): E7223 – E7229. Bibcode:2015PNAS..112E7223C. doi:10.1073/pnas.1519374113. PMC 4702975. PMID 26668394.
- ^ Dassanayake RP, Falkenberg SM, Briggs RE, Tatum FM, Sacco RE (2017-08-21). "Antimicrobial activity of bovine NK-lysin-derived peptides on bovine respiratory pathogen Histophilus somni". PLOS ONE. 12 (8): e0183610. Bibcode:2017PLoSO..1283610D. doi:10.1371/journal.pone.0183610. PMC 5565109. PMID 28827826.
- ^ a b c Harris V, Jackson C, Cooper A (December 2016). "Review of Toxic Epidermal Necrolysis". International Journal of Molecular Sciences. 17 (12): 2135. doi:10.3390/ijms17122135. PMC 5187935. PMID 27999358.
Further reading
[edit]- Krista Conger. Grant to fund research into preventing bioterrorism Archived 2008-06-28 at the Wayback Machine, Stanford Report, November 12, 2003.
- Krensky AM, Clayberger C (August 2005). "Granulysin: a novel host defense molecule". American Journal of Transplantation. 5 (8): 1789–1792. doi:10.1111/j.1600-6143.2005.00970.x. PMID 15996224. S2CID 19946195.
- Walch M, Dotiwala F, Mulik S, Thiery J, Kirchhausen T, Clayberger C, et al. (June 2014). "Cytotoxic cells kill intracellular bacteria through granulysin-mediated delivery of granzymes". Cell. 157 (6): 1309–1323. doi:10.1016/j.cell.2014.03.062. PMC 4090916. PMID 24906149.
- da Silva AP, Unks D, Lyu SC, Ma J, Zbozien-Pacamaj R, Chen X, et al. (May 2008). "In vitro and in vivo antimicrobial activity of granulysin-derived peptides against Vibrio cholerae". The Journal of Antimicrobial Chemotherapy. 61 (5): 1103–1109. doi:10.1093/jac/dkn058. PMC 2664651. PMID 18310138.
- Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. (December 2008). "Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis". Nature Medicine. 14 (12): 1343–1350. doi:10.1038/nm.1884. PMID 19029983. S2CID 2068160.
- Peña SV, Krensky AM (April 1997). "Granulysin, a new human cytolytic granule-associated protein with possible involvement in cell-mediated cytotoxicity". Seminars in Immunology. 9 (2): 117–125. doi:10.1006/smim.1997.0061. PMID 9194222.
- Krensky AM (February 2000). "Granulysin: a novel antimicrobial peptide of cytolytic T lymphocytes and natural killer cells". Biochemical Pharmacology. 59 (4): 317–320. doi:10.1016/S0006-2952(99)00177-X. PMID 10644038.
- Donlon TA, Krensky AM, Clayberger C (1990). "Localization of the human T lymphocyte activation gene 519 (D2S69E) to chromosome 2p12----q11". Cytogenetics and Cell Genetics. 53 (4): 230–231. doi:10.1159/000132938. PMID 2209093.
- Yabe T, McSherry C, Bach FH, Houchins JP (October 1990). "A cDNA clone expressed in natural killer and T cells that likely encodes a secreted protein". The Journal of Experimental Medicine. 172 (4): 1159–1163. doi:10.1084/jem.172.4.1159. PMC 2188624. PMID 2212946.
- Houchins JP, Kricek F, Chujor CS, Heise CP, Yabe T, McSherry C, Bach FH (1993). "Genomic structure of NKG5, a human NK and T cell-specific activation gene". Immunogenetics. 37 (2): 102–107. doi:10.1007/BF00216832. PMID 8423048. S2CID 24229734.
- Peña SV, Hanson DA, Carr BA, Goralski TJ, Krensky AM (March 1997). "Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small, lytic, granule proteins". Journal of Immunology. 158 (6): 2680–2688. doi:10.4049/jimmunol.158.6.2680. PMID 9058801. S2CID 23788667.
- Hanson DA, Kaspar AA, Poulain FR, Krensky AM (May 1999). "Biosynthesis of granulysin, a novel cytolytic molecule". Molecular Immunology. 36 (7): 413–422. doi:10.1016/S0161-5890(99)00063-2. PMID 10449094.
- Kaspar AA, Okada S, Kumar J, Poulain FR, Drouvalakis KA, Kelekar A, et al. (July 2001). "A distinct pathway of cell-mediated apoptosis initiated by granulysin". Journal of Immunology. 167 (1): 350–356. doi:10.4049/jimmunol.167.1.350. PMID 11418670.
- Kitamura N, Koshiba M, Horie O, Ryo R (2002). "Expression of granulysin mRNA in the human megakaryoblastic leukemia cell line CMK". Acta Haematologica. 108 (1): 13–18. doi:10.1159/000063061. PMID 12145461. S2CID 43475285.
- Ma LL, Spurrell JC, Wang JF, Neely GG, Epelman S, Krensky AM, Mody CH (November 2002). "CD8 T cell-mediated killing of Cryptococcus neoformans requires granulysin and is dependent on CD4 T cells and IL-15". Journal of Immunology. 169 (10): 5787–5795. doi:10.4049/jimmunol.169.10.5787. PMID 12421959.
- Ericson KG, Fadeel B, Andersson M, Gudmundsson GH, Gürgey A, Yalman N, et al. (January 2003). "Sequence analysis of the granulysin and granzyme B genes in familial hemophagocytic lymphohistiocytosis". Human Genetics. 112 (1): 98–99. doi:10.1007/s00439-002-0841-0. PMID 12483306. S2CID 23973452.
- Anderson DH, Sawaya MR, Cascio D, Ernst W, Modlin R, Krensky A, Eisenberg D (January 2003). "Granulysin crystal structure and a structure-derived lytic mechanism". Journal of Molecular Biology. 325 (2): 355–365. CiteSeerX 10.1.1.327.5540. doi:10.1016/S0022-2836(02)01234-2. PMID 12488100.
- Gansert JL, Kiessler V, Engele M, Wittke F, Röllinghoff M, Krensky AM, et al. (March 2003). "Human NKT cells express granulysin and exhibit antimycobacterial activity". Journal of Immunology. 170 (6): 3154–3161. doi:10.4049/jimmunol.170.6.3154. PMID 12626573.
- Ogawa K, Takamori Y, Suzuki K, Nagasawa M, Takano S, Kasahara Y, et al. (July 2003). "Granulysin in human serum as a marker of cell-mediated immunity". European Journal of Immunology. 33 (7): 1925–1933. doi:10.1002/eji.200323977. PMID 12884856. S2CID 22280357.
- Kotsch K, Mashreghi MF, Bold G, Tretow P, Beyer J, Matz M, et al. (June 2004). "Enhanced granulysin mRNA expression in urinary sediment in early and delayed acute renal allograft rejection". Transplantation. 77 (12): 1866–1875. doi:10.1097/01.TP.0000131157.19937.3F. PMID 15223905. S2CID 31769483.