Citral
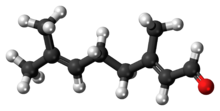
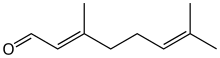 Geranial
| |
| |
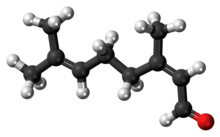
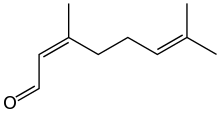 Neral
| |
| |
| Names | |
|---|---|
| IUPAC name
3,7-dimethylocta-2,6-dienal
| |
| Other names
citral
geranialdehyde | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.023.994 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2810 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H16O | |
| Molar mass | 152.24 g/mol |
| Appearance | Pale yellow liquid |
| Odor | Lemon like |
| Density | 0.893 g/cm3 |
| Boiling point | 229 °C (444 °F; 502 K) |
| Vapor pressure | 0.22 mmHg (20 °C) |
| −98.9×10−6 cm3/mol | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H317 | |
| P261, P264, P272, P280, P302+P352, P321, P332+P313, P333+P313, P362, P363, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 91 °C (196 °F; 364 K) |
| Related compounds | |
Related alkenals
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Citral is an acyclic monoterpene aldehyde. Being a monoterpene, it is made of two isoprene units. Citral is a collective term which covers two geometric isomers that have their own separate names; the E-isomer is named geranial (trans-citral; α-citral[2]) or citral A. The Z-isomer is named neral (cis-citral; β-citral[2]) or citral B. These stereoisomers occur as a mixture, often not in equal proportions; e.g. in essential oil of Australian ginger, the neral to geranial ratio is 0.61.[3]
Occurrence
[edit]Citral is present in the volatile oils of several plants, including lemon myrtle (90–98%), Litsea citrata (90%), Litsea cubeba (70–85%), lemongrass (65–85%), lemon tea-tree (70–80%), Ocimum gratissimum (66.5%), Lindera citriodora (about 65%), Calypranthes parriculata (about 62%), petitgrain (36%), lemon verbena (30–35%), lemon ironbark (26%), lemon balm (11%), lime (6–9%), lemon (2–5%), and orange.[4][5][6] Further, in the lipid fraction (essential oil) of Australian ginger (51–71%)[3] Of the many sources of citral, the Australian myrtaceous tree, lemon myrtle, Backhousia citriodora F. Muell. (of the family Myrtaceae), is considered superior.[7]
Uses
[edit]Citral is a precursor in the industrial production of vitamin A, vitamin E, vitamin K.
Citral is also precursor to lycopene, ionone and methylionone.
Fragrances
[edit]Citral has a strong lemon (citrus) scent and is used as an aroma compound in perfumery. It is used to fortify lemon oil. (Nerol, another perfumery compound, has a less intense but sweeter lemon note.) The aldehydes citronellal and citral are considered key components responsible for the lemon note with citral preferred.[7]
It also has pheromonal effects in acari and insects.[8][9]
The herb Cymbopogon citratus has shown promising insecticidal and antifungal activity against storage pests.[10]
Food additive
[edit]Citral is commonly used as a food additive ingredient.[11]
It has been tested (2016) in vitro against the food-borne pathogen Cronobacter sakazakii.[12]
See also
[edit]References
[edit]- ^ Citral, The Merck Index, 12th Edition.
- ^ a b Waghulde, S.; Parmar, P.; Mule, J.; Pashte, D.; Patil, B.; Modhale, N.; Gorde, N.; Kharche, A.; Kale, M. (2020). "Lead Finding from Plant Cymbopogon Citratus with Immunomodulator Potentials through in Silico Methods". Chemistry Proceedings. 3 (1): 77. doi:10.3390/ecsoc-24-08302. ISSN 2673-4583.
- ^ a b Zachariah, T. J.; Parthasarathy, V. A.; Chempakam, B. (2008). Chemistry of spices. Wallingford: CABI. p. 76. ISBN 9781845934057. OCLC 1120264204.
- ^ Fenaroli, G.; Furia, T.E.; Bellanca, N. Handbook of Flavor Ingredients. ISBN 0-87819-532-7.
- ^ Lawless, J. (2 November 1995). The Illustrated Encyclopedia of Essential Oils. Element. ISBN 1-85230-661-0.
- ^ "The Aromatic Plant Project". Archived from the original on 24 November 2019. Retrieved 1 June 2008.
- ^ a b Southwell, Ian (9 July 2021). "Backhousia citriodora F. Muell. (Lemon Myrtle), an Unrivalled Source of Citral". Foods. 10 (7): 1596. doi:10.3390/foods10071596. PMC 8305781. PMID 34359465.
- ^ Kuwahara, Yasumasa; Suzuki, Hiroshi; Matsumoto, Katsuhiko; Wada, Yoshitake (1983). "Pheromone Study on Acarid Mites. XI. Function of Mite Body as Geometrical Isomerization and Reduction of Citral (the Alarm Pheromone)". Applied Entomology and Zoology. 18 (1): 30–39. doi:10.1303/aez.18.30.
- ^ Robacker, D.C.; Hendry, L.B. (1977). "Neral and geranial: components of the sex pheromone of the parasitic wasp, Itoplectis conquisitor". Journal of Chemical Ecology. 3 (5): 563–577. doi:10.1007/BF00989077. S2CID 11568355.
- ^ Dubey, N. K.; Takeya, Koichi; Itokawa, Hideji (1997). "Citral: A cytotoxic principle isolated from the essential oil of Cymbopogon citratus against P388 leukaemia cells". Current Science. 73 (1): 22–24. JSTOR 24098141.
- ^ Liao, Pei-Chun; Yang, Tsung-Shi; Chou, Ju-Ching; Chen, Jie; Lee, Shu-Ching; Kuo, Yueh-Hsiung; Ho, Chen-Lung; Chao, Louis Kuo-Ping (1 December 2015). "Anti-inflammatory activity of neral and geranial isolated from fruits of Litsea cubeba Lour". Journal of Functional Foods. 19: 248–258. doi:10.1016/j.jff.2015.09.034.
- ^ Shi, Chao; Song, Kaikuo; Zhang, Xiaorong; Sun, Yi; Sui, Yue; Chen, Yifei; Jia, Zhenyu; Sun, Huihui; Sun, Zheng; Xia, Xiaodong (14 July 2016). "Antimicrobial Activity and Possible Mechanism of Action of Citral against Cronobacter sakazakii". PLOS ONE. 11 (7): e0159006. Bibcode:2016PLoSO..1159006S. doi:10.1371/journal.pone.0159006. PMC 4945043. PMID 27415761.
External links
[edit]- MSDS Archived 18 August 2007 at the Wayback Machine

