From Wikipedia, the free encyclopedia
Chemical compound
Promestriene Trade names Colpotrofin, Colpotrophine, Delipoderm Other names Estradiol 3-propyl 17β-methyl diether; 17β-Methoxy-3-propoxyestra-1,3,5(10)-triene Routes of Topical Drug class Estrogen ; Estrogen ester ATC code
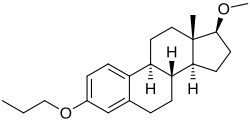
(8R ,9S ,13S ,14S )-17-methoxy-13-methyl-3-propoxy-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a ]phenanthrene
CAS Number PubChem CID ChemSpider UNII CompTox Dashboard (EPA ) ECHA InfoCard 100.049.401 Formula C 22 H 32 O 2 Molar mass −1 3D model (JSmol )
CCCOc1ccc2c(c1)CC[C@@H]3[C@@H]2CC[C@]4([C@H]3CCC4OC)C
InChI=1S/C22H32O2/c1-4-13-24-16-6-8-17-15(14-16)5-7-19-18(17)11-12-22(2)20(19)9-10-21(22)23-3/h6,8,14,18-21H,4-5,7,9-13H2,1-3H3/t18-,19-,20+,21?,22+/m1/s1
N Key:IUWKNLFTJBHTSD-QIKJAYGVSA-N
N (verify)
Promestriene (INN Tooltip International Nonproprietary Name ) (brand names Colpotrofin , Colpotrophine , Delipoderm ), also known as estradiol 3-propyl 17β-methyl diether , is a synthetic estrogen which is used topically in a 1% cream formulation for the treatment of vaginal atrophy in women.[ 1] [ 2] [ 3] [ 4] [ 5] propyl and 17β-methyl diether of estradiol and does not appear to convert into estradiol in the body.[ 1] [ 6] absorbed and appears to have negligible systemic estrogenic effect.[ 1] tropic agent and antiseborrheic .[ 2] pattern hair loss or other conditions of cutaneous androgenization .[ 7] [ 8] France in 1974 and has been marketed in 34 countries worldwide.[ 1] [ 1]
^ a b c d e Del Pup L, Di Francia R, Cavaliere C, Facchini G, Giorda G, De Paoli P, Berretta M (November 2013). "Promestriene, a specific topic estrogen. Review of 40 years of vaginal atrophy treatment: is it safe even in cancer patients?". Anti-Cancer Drugs . 24 (10): 989–998. doi :10.1097/CAD.0b013e328365288e . PMID 24080714 . S2CID 3458356 . ^ a b Ganellin CR, Triggle DJ (21 November 1996). Dictionary of Pharmacological Agents ISBN 978-0-412-46630-4 ^ Index Nominum 2000: International Drug Directory ISBN 978-3-88763-075-1 ^ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition ISBN 978-0-8155-1856-3 ^ Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition ISBN 978-3-7692-2114-5 ^ Santos I, Clissold S (September 2010). "Urogenital disorders associated with oestrogen deficiency: the role of promestriene as topical oestrogen therapy". Gynecological Endocrinology . 26 (9): 644–651. doi :10.3109/09513591003767948 . PMID 20374067 . S2CID 243338 . ^ Orfanos CE, Montagna W, Stüttgen G (6 December 2012). Hair Research: Status and Future Aspects; Proceedings of the First International Congress on Hair Research, Hamburg, March 13th–16, 1979 ISBN 978-3-642-81650-5 ^ Beylot C (1 October 1998). "Menopause, Skin, and Cosmetology" . In Baran R, Maibach HI (eds.). Textbook of Cosmetic Dermatology . CRC Press. pp. 493–. ISBN 978-1-85317-478-0
Estrogens
ER Tooltip Estrogen receptor agonists
Steroidal: Alfatradiol Certain androgens /anabolic steroids (e.g., testosterone , testosterone esters , methyltestosterone , metandienone , nandrolone esters ) (via estrogenic metabolites)
Certain progestins (e.g., norethisterone , noretynodrel , etynodiol diacetate , tibolone )
Clomestrone Cloxestradiol acetate Conjugated estriol Conjugated estrogens Epiestriol Epimestrol Esterified estrogens Estetrol † Estradiol Estradiol esters (e.g., estradiol acetate , estradiol benzoate , estradiol cypionate , estradiol enanthate , estradiol undecylate , estradiol valerate , polyestradiol phosphate , estradiol ester mixtures (Climacteron ))Estramustine phosphate Estriol Estriol esters (e.g., estriol succinate , polyestriol phosphate )Estrogenic substances Estrone Estrone esters
Ethinylestradiol #
Hydroxyestrone diacetate Mestranol Methylestradiol Moxestrol Nilestriol Prasterone (dehydroepiandrosterone; DHEA)
Promestriene Quinestradol Quinestrol Progonadotropins
Antiestrogens
ER Tooltip Estrogen receptor antagonistsSERMs Tooltip selective estrogen receptor modulators /SERDs Tooltip selective estrogen receptor downregulators )Aromatase inhibitors Antigonadotropins
Androgens /anabolic steroids (e.g., testosterone , testosterone esters , nandrolone esters , oxandrolone , fluoxymesterone )D2 receptor antagonists (prolactin releasers) (e.g., domperidone , metoclopramide , risperidone , haloperidol , chlorpromazine , sulpiride )GnRH agonistsleuprorelin , goserelin )GnRH antagonistscetrorelix , elagolix )Progestogens (e.g., chlormadinone acetate , cyproterone acetate , gestonorone caproate , hydroxyprogesterone caproate , medroxyprogesterone acetate , megestrol acetate ) Others
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin , pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown