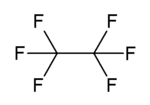
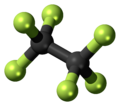
Hexafluoroethane
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Hexafluoroethane | |||
| Other names
Carbon hexafluoride, 1,1,1,2,2,2-Hexafluoroethane, Perfluoroethane, Ethforane, Halocarbon 116, PFC-116, CFC-116, R-116, Arcton 116, Halon 2600, UN 2193
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.855 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2193 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2F6 | |||
| Molar mass | 138.01 g.mol−1 | ||
| Appearance | Colorless odorless gas | ||
| Density | 5.734 kg.m−3 at 24 °C | ||
| Melting point | −100.6 °C (−149.1 °F; 172.6 K) | ||
| Boiling point | −78.2 °C (−108.8 °F; 195.0 K) | ||
| 0.0015% | |||
| log P | 2 | ||
Henry's law
constant (kH) |
0.000058 mol.kg−1.bar−1 | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Supplementary data page | |||
"ARMADURA Z29 HELMET ARMOR Z29" by OSCAR CREATIVO | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Hexafluoroethane is the perfluorocarbon counterpart to the hydrocarbon ethane. It is a non-flammable gas negligibly soluble in water and slightly soluble in methanol. It is an extremely potent and long-lived greenhouse gas.
Physical properties
[edit]Hexafluoroethane's solid phase has two polymorphs. In the scientific literature, different phase transition temperatures have been stated. The latest works assign it at 103 K (−170 °C). Below 103 K it has a slightly disordered structure, and over the transition point, it has a body centered cubic structure.[1] The critical point is at 19.89 °C (293.04 K) and 30.39 bar.[2]
Table of densities:
| State, temperature | Density (kg.m−3) |
|---|---|
| liquid, −78.2 °C | 16.08 |
| gas, −78.2 °C | 8.86 |
| gas, 15 °C | 5.84 |
| gas, 20.1 °C | 5.716 |
| gas, 24 °C | 5.734 |
Vapor density is 4.823 (air = 1), specific gravity at 21 °C is 4.773 (air = 1) and specific volume at 21 °C is 0.1748 m3/kg.
Uses
[edit]Hexafluoroethane is used as a versatile etchant in semiconductor manufacturing. It can be used for selective etching of metal silicides and oxides versus their metal substrates and also for etching of silicon dioxide over silicon. The primary aluminium and the semiconductor manufacturing industries are the major emitters of hexafluoroethane using the Hall-Héroult process.
Together with trifluoromethane it is used in refrigerants R508A (61%) and R508B (54%).
It is used as a tamponade to assist in retinal reattachment following vitreoretinal surgery.[3]
Environmental effects
[edit]

Due to the high energy of C−F bonds, hexafluoroethane is nearly inert and thus acts as an extremely stable greenhouse gas, with an atmospheric lifetime of 10,000 years (other sources: 500 years).[4] It has a global warming potential (GWP) of 9200 and an ozone depletion potential (ODP) of 0. Hexafluoroethane is included in the IPCC list of greenhouse gases.
Hexafluoroethane did not exist in significant amounts in the environment prior to industrial-scale manufacturing. Atmospheric concentration of hexafluoroethane reached 3 pptv at the start of the 21st century.[5] Its absorption bands in the infrared part of the spectrum cause a radiative forcing of about 0.001 W/m2.
Health risks
[edit]Due to its high relative density, it gathers in low-lying areas, and at high concentrations it can cause asphyxiation.
See also
[edit]References
[edit]- ^ Zeng, S.X.; Simmons, R.O.; Timms, D.N.; Evans, A.C. (1999). "Dynamics and structure of solid hexafluoroethane". Journal of Chemical Physics. 110 (3): 1650–61. Bibcode:1999JChPh.110.1650Z. doi:10.1063/1.477806.
- ^ Helmut Schan: Handbuch der reinsten Gase. Springer, 2005, ISBN 978-3-540-23215-5, S. 307.
- ^ Andreas Kontos; James Tee; Alastair Stuart; Zaid Shalchi; Tom H Williamson (2016). "Duration of intraocular gases following vitreoretinal surgery". Graefes Arch Clin Exp Ophthalmol. 255 (2): 231–236. doi:10.1007/s00417-016-3438-3. PMID 27460279. S2CID 23629379.
- ^ "Perfluoroethane CASRN: 76-16-4". TOXNET Toxicology Data Network. National Library of Medicine. 2016-10-25.
- ^ "Climate Change 2001: The Scientific Basis". Archived from the original on 2007-06-15. Retrieved 2007-06-02.
- Bozin SE, et al. (1968). "Growth of ionization currents in carbon tetrafluoride and hexafluoroethane". J. Phys. D: Appl. Phys. 1 (3): 327–334. Bibcode:1968JPhD....1..327B. doi:10.1088/0022-3727/1/3/309. S2CID 250891553.
External links
[edit]- Purification process of hexafluoroethane
- Protocol for measurement of tetrafluoromethane and hexafluoroethane from primary aluminium production
- De Maré, G.R.; Panchenko, Yu. N. (March 2006). "Ab initio vibrational analysis of hexafluoroethane C2F6". Journal of Structural Chemistry. 47 (2): 232–240. doi:10.1007/s10947-006-0291-y. S2CID 96363970.
- Protocol for Measurement of Tetrafluoromethane (CF
4) and Hexafluoroethane (C
2F
6) Emissions from Primary Aluminum Production - Thermochemistry data table at chemnet.ru