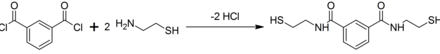BDTH2
| |
| |
| Names | |
|---|---|
| IUPAC name
N,N′-bis(2-mercaptoethyl)isophthalamide
| |
| Other names
BDET; BDTH2; BDETH2; N,N′-Bis(2-mercaptoethyl)-1,3-benzenedicarboxamide; N1,N3-bis(2-mercaptoethyl)isophthalamide; NBMI;
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | 1,3-benzenediamidoethanethiol |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H16N2O2S2 | |
| Molar mass | 284.39 g·mol−1 |
| Density | 1.23 g/mL |
| Melting point | 132 to 135 °C (270 to 275 °F; 405 to 408 K) [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
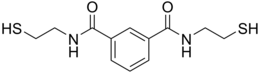
BDTH2 (also called BDET and BDETH2; trade names B9, MetX and OSR#1) is an organosulfur compound that is used as a chelation agent.[2] It is a colourless solid. The molecule consists of two thiol groups and linked via a pair of amide groups.[3]
Preparation
The compound was reported in about 1994 after a search for chelating agents selective for mercury. It was licensed in 2006 to CTI Science with the long-term goal of using BDTH2 in mercury poisoning.[4] This compound is prepared by treating isophthaloyl dichloride with two equiv of cysteamine:[1][2]
Use
Environmental remediation
BDTH2 can be used to chelate heavy metals like lead, cadmium, copper, manganese, zinc, iron, and mercury from ground water, coal tailings, gold ore, waste water of battery-recycling plants, and contaminated soil.[2]
BDTH2 appears to bind mercury more strongly than do other chelators. The mercury-BDT complex does not break down even at high pH and in the presence of cyanides, as in waste water of gold mines. The particular stability of the mercury bond can be attributed to the linear position of the two thiols.[5] The company Covalent Research Technologies had investigated BDTH2 for the removal of mercury from flue gas without success.[4]
Clinical use
Animal experiments with inorganic mercury showed, that BDTH2 effectively binds mercury in the body, and the resulting mercury derivative is excreted in the feces. Experimental animals showed no signs of poisoning. It is unclear, how the BDTH2-mercury-chelate behaves in the long term. BDTH2 is lipophilic, as opposed to DMPS and DMSA and thus can cross lipid membranes including the blood-brain-barrier and enters the bone marrow.[4] In animal experiments, the amount of mercury in brain tissue was not increased, but also not decreased(can anyone provide a reference for the material/data/research from which this conclusion was drawn?). However, there are indications that the BDTH2-mercury-compound moves into adipose tissue.[3] It is unknown, how BDTH2 works with methyl-mercury.
BDTH2 appears to bind copper and zinc in vivo only weakly. In contrast DMPS und DMSA bind these ions more strongly. Its affinity is low for other "hard" ions, e.g. Ca2+, Mg2+, Na+, and K+.[3]
Until July 2010 CTI Science sold BDTH2 under the name OSR#1 as a nutritional supplement.[6] Since OSR#1 didn't fulfill criteria of a nutritional supplement, its sale was stopped under pressure of the U.S. Food and Drug Administration.[7] In January 2012 BDTH2 was designated by the European Commission as an orphan drug, which guarantees CTI Science 10 years of exclusive marketing rights.[8] The U.S. FDA in April 2012 also designated the compound as an orphan drug[9]
Potential applications
Like most thiols, BDTH2 binds to mercury salts to form thiolate complexes. In principle, it could be used to remove mercury from water for industrial applications under a wide range of conditions, including the high pH and cyanide of the effluent from gold mining. In industrial use, BDTH2 is easy to make and can be used either as-is or in the form of sodium or potassium salts that are more soluble in water.[1]
BDTH2 binds to mercury with a strong, nonpolar covalent bond within a water-insoluble organic framework. The resulting BDT–Hg precipitate is stable, and leaches mercury only under highly acidic or basic conditions. BDTH2 also binds to other elements, including arsenic, cadmium, copper, lead, and selenium.[1] It is effective and economical for removing small traces of mercury from polluted soil, as the precipitate is inert and can be left in the soil after treatment.[10]
Dietary supplement and controversy
BDTH2 had been marketed under the name OSR#1 as a dietary supplement for treatment of autism.[11] The U.S. Food and Drug Administration determined that BDTH2 is a drug rather than a supplement and issued a warning,[12][13] resulting in its removal from the market.[14] The main proponent of the compound, Dr. Boyd Haley, was chairman of the department of chemistry where research is also conducted on the utility of this compound for remediation of heavy metal pollution.[1][11]
See also
References
- ^ a b c d e Blue LY, Jana P, Atwood DA. Aqueous mercury precipitation with the synthetic dithiolate, BDTH2. Fuel. June 2010;89(6):1326-1330. doi:10.1016/j.fuel.2009.10.031.
- ^ a b c Blue LY, Immobilizatin of mercury and arsenic through covalent thiolate bonding for the purpose of environmental remediation, Dissertation, University of Kentucky, 2010
- ^ a b c Clarke D, Efficacy of a Novel Chelator for Mercury Chelation and Distribution, Dissertation, Arkansas State University, December 2012
- ^ a b c Mullin, Rick (3 March 2014). "A mercury chelator". C&EN Online. 92 (9): 18–19.
- ^ Lisa Y. Blue, Mike A. Van Aelstyn, Matthew Matlock, David A. Atwood, Low-level mercury removal from groundwater using a synthetic chelating ligand, Water Research, Vol. 42, Issues 8-9, April 2008, pp2025-2028
- ^ Forrest Health Online:"Archived copy". Archived from the original on 2010-12-23. Retrieved 2014-07-15.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: archived copy as title (link) - ^ Warning letter CIN-10-107927-14 17 June 2010 FDA to CTI Science Inc.
- ^ EU/3/11/944 (PDF; 112 kB) NBMI as orphan drug by the EC
- ^ http://www.accessdata.fda.gov/scripts/opdlisting/oopd/OOPD_Results_2.cfm?Index_Number=367312
- ^ Blue LY, Van Aelstyn MA, Matlock M, Atwood DA. Low-level mercury removal from groundwater using a synthetic chelating ligand. Water Res. 2008;42(8–9):2025–8. doi:10.1016/j.watres.2007.12.010.
- ^ a b Tsouderos T. OSR#1: industrial chemical or autism treatment? Chicago Tribune. 2010-01-17.
- ^ Tsouderos, Trine (June 23, 2010). "FDA warns maker of product used as alternative autism treatment". Chicago Tribune. Archived from the original on August 26, 2010. Retrieved July 30, 2010.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Warning Letter CIN-10-107927-14". Inspections, Compliance, Enforcement, and Criminal Investigations. U.S Department of Health and Human Services / Food and Drug Administration. June 17, 2010. Archived from the original on 27 June 2010. Retrieved June 28, 2010.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Tsouderos, Trine (July 26, 2010). "Controversial supplement to come off shelves". Chicago Tribune. Archived from the original on 30 July 2010. Retrieved July 30, 2010.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)