2-Aminobenzaldehyde
Appearance

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Aminobenzaldehyde | |
| Systematic IUPAC name
2-Aminobenzenecarbaldehyde | |
| Other names
ortho-Aminobenzaldehyde
2-Formylaniline | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.687 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H7NO | |
| Molar mass | 121.139 g·mol−1 |
| Appearance | yellow solid |
| Melting point | 32–34 °C (90–93 °F; 305–307 K) |
| good | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Aminobenzaldehyde is an organic compound with the formula C6H4(NH2)CHO. It is one of three isomers of aminobenzaldehyde. It is a low-melting yellow solid that is soluble in water.
Preparation and reactions
It is usually prepared by reduction of 2-nitrobenzaldehyde with iron[1] or iron(II) sulfate.[2] The compound is unstable with respect to condensation with itself.
It is used to prepare quinolines by the Friedländer synthesis:
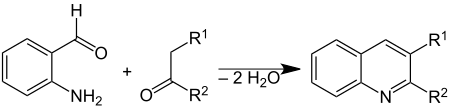
By template reactions, it also forms trimeric and tetrameric condensation products that have been studied as ligands.

References
- ^ Chen Zhang; Chandra Kanta De; Daniel Seidel (2012). "o-Aminobenzaldehyde, Redox-Neutral Aminal Formation and Synthesis of Deoxyvasicinone". Org. Synth. 89: 274. doi:10.15227/orgsyn.089.0274.
- ^ Lee Irvin Smith; J. W. Opie (1948). "o-Aminobenzaldehyde". Org. Synth. 28: 11. doi:10.15227/orgsyn.028.0011.
- ^ Fleischer, E. B.; Klem, E. (1965). "The Structure of a Self-Condensation Product of o-Aminobenzaldehyde in the Presence of Nickel Ions". Inorganic Chemistry. 4: 637–642. doi:10.1021/ic50027a008.
