Organoboron chemistry

Organoboron chemistry or organoborane chemistry studies organoboron compounds, also called organoboranes. These chemical compounds combine boron and carbon; typically, they are organic derivatives of borane (BH3), as in the trialkyl boranes.[1][2]
Organoboranes and -borates enable many chemical transformations in organic chemistry — most importantly, hydroboration and carboboration. Most reactions transfer a nucleophilic boron substituent to an electrophilic center either inter- or intramolecularly. In particular, α,β-unsaturated borates and borates with an α leaving group are highly susceptible to intramolecular 1,2-migration of a group from boron to the electrophilic α position. Oxidation or protonolysis of the resulting organoboranes generates many organic products, including alcohols, carbonyl compounds, alkenes, and halides.[3]
Properties of the B-C bond
[edit]The C-B bond has low polarity (electronegativity 2.55 for carbon and 2.04 for boron). Alkyl boron compounds are in general stable, though easily oxidized.
Boron often forms electron-deficient compounds without a full octet, such as the triorganoboranes. These compounds are strong electrophiles, but typically too sterically hindered to dimerize. Electron donation from vinyl and aryl groups can lend the C-B bond some double bond character.
Classes of organoboron compounds
[edit]Organoboranes and hydrides
[edit]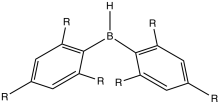
The most-studied class of organoboron compounds has the formula BRnH3−n. These compounds are catalysts, reagents, and synthetic intermediates. The trialkyl and triaryl derivatives feature a trigonal-planar boron center that is typically only weakly Lewis acidic. Except a few bulky derivatives, the hydrides (n = 1 or 2) dimerize, like diborane itself. Trisubstituted derivatives, e.g. triethylboron, are monomers.[5]
Monoalkyl boranes are relatively rare. When the alkyl group is small, such as methyl, the monoalkylboranes tend to redistribute to give mixtures of diborane and di- and trialkylboranes. Monoalkylboranes typically exist as dimers of the form [RBH2]2. One example is thexylborane (ThxBH2), produced by the hydroboration of tetramethylethylene:[6] A chiral example is monoisopinocampheylborane. Although often written as IpcBH2, it is a dimer [IpcBH2]2. It is obtained by hydroboration of (−)‐α‐pinene with borane dimethyl sulfide.[7]
Dialkylboranes are also rare for small alkyl groups. One common way of preparing them is the reduction of dialkylhalogenoboranes with metal hydrides.[8] An important synthetic application using such dialkylboranes, such as diethylborane, is the transmetallation of the organoboron compounds to form organozinc compounds.[9][10]
Some diaryl and dialkylboranes are well known. Dimesitylborane is a dimer (C6H2Me3)4B2H2). It reacts only slowly with simple terminal alkenes. It adds to alkynes to give alkenylboranes.[11] Among hindered dialkylboranes is disiamylborane, abbreviated Sia2BH. It also is a dimer. Owing to its steric bulk, it selectively hydroborates less hindered, usually terminal alkenes in the presence of more substituted alkenes.[12] Disiamylborane must be freshly prepared as its solutions can only be stored at 0 °C for a few hours. Dicyclohexylborane Chx2BH exhibits improved thermal stability than Sia2BH. A versatile dialkylborane is 9-BBN. Also called "banana borane", it exists as a dimer. It can be distilled without decomposition at 195 °C (12mm Hg). Reactions with 9-BBN typically occur at 60–80 °C, with most alkenes reacting within one hour. Tetrasubstituted alkenes add 9-BBN at elevated temperature. Hydroboration of alkenes with 9-BBN proceeds with excellent regioselectivity. It is more sensitive to steric differences than Sia2BH, perhaps because of it rigid C8 backbone. 9-BBN is more reactive towards alkenes than alkynes.[13]
Borinic and boronic acids and esters (BRn(OR)3-n)
[edit]Compounds of the type BRn(OR)3-n are called borinic esters (n = 2), boronic esters (n = 1), and borates (n = 0). Boronic acids are key to the Suzuki reaction. Trimethyl borate, debatably not an organoboron compound, is an intermediate in sodium borohydride production.
Boron clusters
[edit]Boron is renowned for cluster species, e.g. dodecaborate [B12H12]2-. Such clusters have many organic derivatives. One example is [B12(CH3)12]2- and its radical derivative [B12(CH3)12]−.[14] Related cluster compounds with carbon vertices are carboranes; the best known is orthocarborane, C2B10H12. Carboranes have few commercial applications. Anionic derivatives such as [C2B9H11]2−, called dicarbollides, ligate similarly to cyclopentadienide.
Bora-substituted aromatic compounds
[edit]In borabenzene, boron replaces one CH center in benzene. Borabenzene and derivatives invariably appear as adducts, e.g., C5H5B-pyridine.
The cyclic compound borole, a structural analog of pyrrole, has not been isolated, but substituted derivatives (boroles) are known.
The cyclic compound borepin is aromatic.
Boryl compounds
[edit]Organometallic compounds with metal-boron bonds (M–BR2) are boryl complexes, corresponding to the notional boryl anion R2B−. Related ligands are borylenes (M–B(R)–M).
Strong bases do not deprotonate boranes R2BH. Instead these reactions afford the octet-complete adduct R2HB-base.[15]
Compounds isoelectronic with the N-heterocyclic carbenes are known The unusual compound was prepared by reduction of a boron-bromide precursor:[16][17]
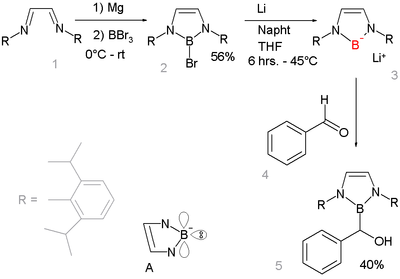
Boryl radicals
[edit]Alkylideneboranes
[edit]Alkylideneboranes (RB=CRR) with a boron–carbon double bond are rare. One example is borabenzene. The parent compound, HB=CH2, can be detected at low temperature. The derivative CH3B=C(SiMe3)2 is fairly stable, but prone to cyclodimerisation.[18]
NHC adducts of boron
[edit]NHCs and boranes form stable NHC-borane adducts.[19] Triethylborane adducts can be synthesised directly from the imidazolium salt and lithium triethylborohydride.
Diborenes
[edit]Boron-boron double bonds are rare. One example is the diborene (RHB=BHR):[20][21]
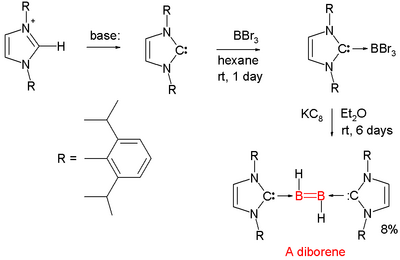
Each boron atom has an attached proton and is coordinated to a NHC carbene. The parent structure with the additional carbene ligands is diborane(2).[22][23]
A reported diboryne is based on similar chemistry.
Synthesis
[edit]From Grignard reagents
[edit]Simple organoboranes such as triethylborane or tris(pentafluorophenyl)boron can be prepared from trifluoroborane (in ether) and the ethyl or pentafluorophenyl Grignard reagent. Further carbanion addition will effect a borate (R4B−).
Boronic acids RB(OH)2 react with potassium bifluoride K[HF2] to form trifluoroborate salts K[RBF3],[24] precursors to nucleophilic alkyl and aryl boron difluorides, ArBF2:[25]
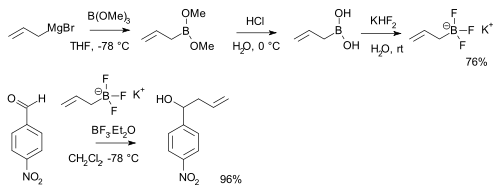
From alkenes
[edit]In hydroboration, alkenes insert into borane B-H bonds, with anti-Markovnikov stereochemistry. Hydroboration occurs stereospecifically syn — on the same alkene face. The transition state for this concerted reaction can be visualized as a square with the corners occupied by carbon, carbon, hydrogen and boron, maximizing overlap between the olefin p-orbitals and the empty boron orbital.
Hydroboration with borane (BH3) equivalents converts only 33% of the starting olefin to product — boron-containing byproducts consume the remainder. The chelate effect improves that ratio for cyclic boron-containing reagents. One common cyclic organoboron reagent is 9-BBN.[26][27]
By borylation
[edit]Metal-catalyzed borylation reactions produce an organoboron compound from aliphatic or aromatic C-H sigma bonds via a transition-metal catalyst. A common reagent is bis(pinacolato)diboron.
From other boron compounds
[edit]Carbon monoxide reacts with alkylboranes to form an unstable borane carbonyl. Then an alkyl substituent migrates from boron to the carbonyl carbon. For example, homologated primary alcohols result from organoboranes, carbon monoxide, and a reducing agent (here, sodium borohydride):[28]

Alkenylboranes
[edit]Alkynylboranes attack electrophiles to give trans alkenylboranes,[29] as in the first step of this olefin synthesis:
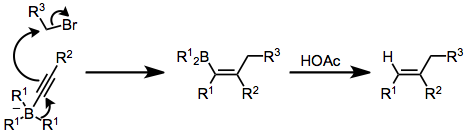
Reactions
[edit]
The key property of organoboranes (R3B) and borates (R4B−, generated via addition of R− to R3B) is their susceptibility to reorganization. These compounds possess boron–carbon bonds polarized toward carbon. The boron-attached carbon is nucleophilic;[30] in borates, the nucleophicity suffices for intermolecular transfer to an electrophile.[31][3]
Boranes alone are generally not nucleophilic enough to transfer an R group intermolecularly. Instead, the group 1,2-migrates to an electrophilic carbon attached to boron, especially if that carbon is unsaturated or bears a good leaving group:[31]
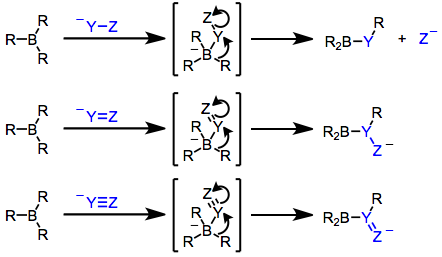
An organic group's migration propensity depends on its ability to stabilize negative charge: alkynyl > aryl ≈ alkenyl > primary alkyl > secondary alkyl > tertiary alkyl.[32] Bis(norbornyl)borane and 9-BBN are often hydroboration reagents for this reason — only the hydroborated olefin is likely to migrate upon nucleophilic activation.
Migration retains configuration at the migrant carbon[33] and inverts it at the (presumably sp3-hybridized) terminus.[34] The resulting reorganized borane can then be oxidized or protolyzed to a final product.
Protonolysis
[edit]Organoboranes are unstable to Brønsted–Lowry acids, deboronating in favor of a proton. Consequently, organoboranes are easily removed from an alkane or alkene substrate, as in the second step of this olefin synthesis:[29]

Addition to halocarbonyls
[edit]α-Halo enolates are common nucleophiles in borane reorganization. After nucleophilic attack at boron, the resulting ketoboronate eliminates the halogen and tautomerizes to a neutral enolborane. A functionalized carbonyl compound then results from protonolysis,[35] or quenching with other electrophiles:

Because the migration is stereospecific, this method synthesizes enantiopure α-alkyl or -aryl ketones.[36]
α-Haloester enolates add similarly to boranes, but with lower yields:[37]
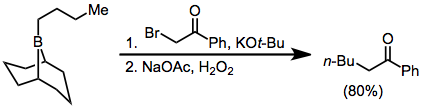
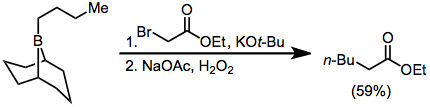
Diazoesters and diazoketones remove the requirement for external base.[38] α,α'-Dihalo enolates react with boranes to form α-halo carbonyl compounds that can be further functionalized at the α position.[39]
Addition to carbonyls
[edit]In allylboration, an allylborane adds across an aldehyde or ketone with an allylic shift, and can then be converted to a homoallylic alcohol during workup. The reaction is much slower with ketones than aldehydes.[40] For example, in Nicolaou's epothilones synthesis, asymmetric allylboration (with an allylborane derived from chiral alpha-pinene) is the first step in a two-carbon homologation to acetogenin:[41]
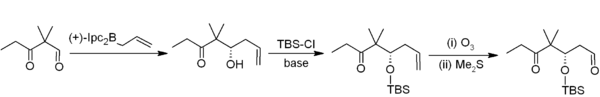
Trifluoroborate salts are stabler than boronic acids and selectively alkylate aldehydes:[42]

Oxygenation
[edit]The hydroboration-oxidation reaction pair oxidizes the borane to an alcohol with hydrogen peroxide or to a carbonyl group with chromium oxide.
Oxidation of an alkenylborane gives an boron-free enol.[43]
Halogenation
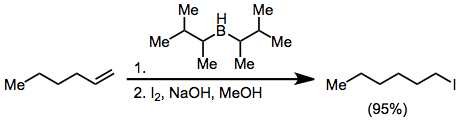
[edit]Organoborane activation with hydroxide or alkoxide and treatment with X2 yields haloalkanes. With excess base, two of the three alkyl groups attached to the boron atom may convert to halide, but disiamylborane permits only halogenation of the hydroborated olefin:[44]
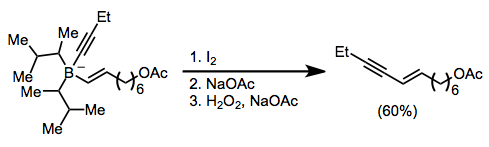
Treatment of an alkenylborane with iodine or bromine induces migration of a boron-attached organic group. Alkynyl groups migrate selectively, forming enynes after treatment with sodium acetate and hydrogen peroxide:[45]
Transmetalation and coupling
[edit]Organoboron compounds also transmetalate easily, especially to organopalladium compounds. In the Suzuki reaction, an aryl- or vinyl-boronic acid couples to an aryl- or vinyl-halide through a palladium(0) complex catalyst:[46]
Reducing agents
[edit]Borane hydrides such as 9-BBN and L-selectride (lithium tri(sec-butyl)borohydride) are reducing agents. An asymmetric catalyst for carbonyl reductions is the CBS catalyst, which relies on boron coordination to the carbonyl oxygen.
Other synthetic applications
[edit]Alcohols
[edit]Homologated primary alcohols result from the treatment of organoboranes with carbon monoxide and a hydride:[47]

Tertiary alcohols with two identical groups attached to the alcohol carbon may be synthesized through an alkynylborane double migration:[43]

Carbonyl groups
[edit]Organoborates anions reductively eliminate against acyl halides. Here, the borate was generated from tri(cyclopentyl)borane and phenyllithium; the three cyclopentyl groups do not significantly migrate:[48]

Applications
[edit]Organoboron chemistry is mainly of commercial value in the pharmaceutical industry.
Triethylborane was used to ignite the JP-7 fuel of the Pratt & Whitney J58 variable cycle engines powering the Lockheed SR-71 Blackbird.
Organoboron compounds have long been discussed for use as boron delivery agents in neutron capture therapy of cancer.[49]
References
[edit]Further reading
[edit]- Thomas, Susan E. The Roles of Boron and Silicon. Oxford Chemistry Primers No. 1; 1991.
Footnotes
[edit]- ^ Thomas 1991
- ^ Elschenbroich, Christoph. Organometallics 3rd Ed. 2006 ISBN 3-527-29390-6 – Wiley-VCH, Weinheim
- ^ a b Negishi E.-I.; Idacavage, M. J. Org. React. 1985, 33, 1. doi:10.1002/0471264180.or033.01
- ^ Bartlett, Ruth A.; Dias, H. V. Rasika; Olmstead, Marilyn M.; Power, Philip P.; Weese, Kenneth J. (1990). "Synthesis of the monomeric HBtrip2 (Trip - 2,4,6-iso-Pr3C6H2) and the x-ray crystal structures of [HBMes2]2 (Mes = 2,4,6,-Me3C6H2) and HBtrip2". Organometallics. 9: 146–150. doi:10.1021/om00115a023.
- ^ Brown, H. C. Organic Syntheses via Boranes John Wiley & Sons, Inc. New York: 1975. ISBN 0-471-11280-1.
- ^ Negishi, Ei-Ichi; Brown, Herbert C. (1974). "Thexylborane-A Highly Versatile Reagent for Organic Synthesis via Hydroboration". Synthesis. 1974 (2): 77–89. doi:10.1055/s-1974-23248.
- ^ Dhar, Raj K.; Josyula, Kanth V. B.; Todd, Robert; Gagare, Pravin D.; Ramachandran, Veeraraghavan (2001). "Diisopinocampheylborane". Encyclopedia of Reagents for Organic Synthesis. pp. 1–10. doi:10.1002/047084289X.rd248.pub3. ISBN 9780470842898.
- ^ Brown, H. C.; Kulkarni, S. U. (1981). "Organoboranes: XXV. Hydridation of dialkylhaloboranes. New practical syntheses of dialkylboranes under mild conditions". Journal of Organometallic Chemistry. 218: 299. doi:10.1016/S0022-328X(00)81001-3.
- ^ Boudier, A.; Hupe, E.; Knochel, P. (2000). "Highly Diastereoselective Synthesis of Monocyclic and Bicyclic Secondary Diorganozinc Reagents with Defined Configuration". Angewandte Chemie International Edition. 39 (13): 2294–2297. doi:10.1002/1521-3773(20000703)39:13<2294::AID-ANIE2294>3.0.CO;2-J. PMID 10941068.
- ^ Hupe, E.; Knochel, P. (2001). "Stereoselective Synthesis of Secondary Organozinc Reagents and Their Reaction with Heteroatomic Electrophiles". Organic Letters. 3 (1): 127–30. doi:10.1021/ol0068400. PMID 11429854.
- ^ Pelter, A.; Singaram, S.; Brown, H. C. (1983). "The Dimesitylboron Group in Organic Chemistry. 6 Hydroborations with dimesitylborane". Tetrahedron Letters. 24 (13): 1433. doi:10.1016/S0040-4039(00)81675-5.
- ^ Dodd, D.S.; Ochlschlager, A. C. (1992). "Synthesis of inhibitors of 2,3-oxidosqualene-lanosterol cyclase: conjugate addition of organocuprates to N-(carbobenzyloxy)-3-carbomethoxy-5,6-dihydro-4-pyridone". Journal of Organic Chemistry. 57 (10): 2794. doi:10.1021/jo00036a008.
- ^ Dhillon, R. S. (2007). Hydroboration and Organic Synthesis : 9-Borabicyclo [3.3.1] Nonane (9-BBN). Springer.
- ^ Grimes, R. N. (2016). Carboranes (3rd ed.). New York: Academic Press. ISBN 9780128019054.
- ^ Hall, Dennis G. Boronic Acids: Preparation, Applications in Organic Synthesis and Medicine. ISBN 3-527-30991-8
- ^ Segawa Yasutomo; Yamashita Makoto; Nozaki Kyoko (2006). "Boryllithium: Isolation, Characterization, and Reactivity as a Boryl Anion". Science. 314 (5796): 113–115. Bibcode:2006Sci...314..113S. doi:10.1126/science.1131914. PMID 17023656. S2CID 21040230.
- ^ Halford, Bethany. "Attacks: Electropositive element pressed into action as nucleophilic boryllithium" Chemical & Engineering News 2006; Volume 84 (41): 11
- ^ Paetzold, Peter; Englert, Ulli; Finger, Rudolf; Schmitz, Thomas; Tapper, Alexander; Ziembinski, Ralf (2004). "Reactions at the Boron-Carbon Double Bond of Methyl(methylidene)boranes". Z. Anorg. Allg. Chem. 630 (4): 508–518. doi:10.1002/zaac.200300396.
- ^ Curran, D. P.; Solovyev, A.; Makhlouf, Brahmi M.; Fensterbank, L.; Malacria, M.; Lacôte, E. (2011). "Synthesis and Reactions of N-Heterocyclic Carbene Boranes". Angewandte Chemie International Edition. 50 (44): 10294–10317. doi:10.1002/anie.201102717. PMID 21898724.
- ^ Wang Yuzhong; Quillian, Brandon; Wei Pingrong; Chaitanya, S. Wannere; Xie Yaoming; King, R. Bruce; Schaefer III, Henry F.; v. R. Schleyer, Paul; Robinson, Gregory H. (2007). "A Stable Neutral Diborene Containing a B=B Double Bond". J. Am. Chem. Soc. 129 (41): 12412–12413. doi:10.1021/ja075932i. PMID 17887683.
- ^ Dagani, Ron. "Neutral Diborene Is A First" Chemical & Engineering News October 1, 2007 Volume 85, Number 40 p. 10
- ^ Braunschweig, Holger; Dewhurst, Rian D. (2013-03-25). "Single, Double, Triple Bonds and Chains: The Formation of Electron-Precise B-B Bonds". Angewandte Chemie International Edition. 52 (13): 3574–3583. doi:10.1002/anie.201208189. ISSN 1521-3773. PMID 23362015.
- ^ Arrowsmith, Merle; Braunschweig, Holger; Stennett, Tom E. (2017-01-02). "Formation and Reactivity of Electron-Precise B−B Single and Multiple Bonds" (PDF). Angewandte Chemie International Edition. 56 (1): 96–115. doi:10.1002/anie.201610072. ISSN 1521-3773. PMID 27860056. Archived (PDF) from the original on July 22, 2018.
- ^ Vedejs, E.; Chapman, R. W.; Fields, S. C.; Lin, S.; Schrimpf, M. R. (1995). "Conversion of Arylboronic Acids into Potassium Aryltrifluoroborates: Convenient Precursors of Arylboron Difluoride Lewis Acids". J. Org. Chem. 60 (10): 3020–3027. doi:10.1021/jo00115a016.
- ^ Molander, Gary A.; Canturk, Belgin (2009). "Organotrifluoroborates and Monocoordinated Palladium Complexes as Catalysts—A Perfect Combination for Suzuki–Miyaura Coupling". Angew. Chem. Int. Ed. 48 (49): 9240–9261. doi:10.1002/anie.200904306. PMC 2917751. PMID 19899086.
- ^ Carey, F. A.; Sundberg, R. J. Advanced Organic Chemistry. ISBN 0-306-41088-5
- ^ Jacob III, P.; Brown, H. C. J. Org. Chem. 1977, 42, 579.
- ^ Rathke, M. W.; Brown, H. C. J. Am. Chem. Soc. 1967, 89, 2740.
- ^ a b Corey, E. J.; Ravindranathan, T. J. Am. Chem. Soc. 1972, 94, 4013.
- ^ Allred, A. L.; Rochow, E. G. J. Inorg. Nucl. Chem. 1958, 5, 264.
- ^ a b Negishi E.-I. J. Organometal. Chem. 1976, 108, 281.
- ^ Miyaura M.; Sasaki N.; Itoh M.; Suzuki A. Tetrahedron Lett. 1977, 173.
- ^ Zweifel, G. in Aspects of Mechanism and Organometallic Chemistry, J. H. Bewster, Ed., Plenum, 1978, p. 229.
- ^ Midland, M. M.; Zolopa, A. R.; Halterman, R. L. J. Am. Chem. Soc. 1979, 101, 248.
- ^ Brown, H. C.; Rogic M. M.; Nambu H.; Rathke, M. W. J. Am. Chem. Soc. 1969, 91, 2147.
- ^ Nesmeyanov, A. N.; Sokolik, R. A. The Organic Compounds of Boron, Aluminium, Gallium, Indium, and Thallium, North-Holland, Amsterdam, 1967.
- ^ Brown, H. C.; Rogic, M. M.; Rathke, M. W.; Kabalka, G. W. J. Am. Chem. Soc. 1968, 90, 818.
- ^ Hooz, J.; Gunn, D. M. J. Am. Chem. Soc. 1969, 91, 6195.
- ^ Pasto, D. J.; Wojtkowski, P. W. J. Org. Chem. 1971, 36, 1790.
- ^ Lachance, H.; Hall, D. (2008). "Allylboration of Carbonyl Compounds". Organic Reactions. Vol. 73. p. 1. doi:10.1002/0471264180.or073.01. ISBN 978-0471264187.
- ^ Nicolaou, K. C.; Sarabia, F.; Ninkovic, S.; Finlay, M. R. V.; Boddy, C. N. C. (1998). "Probing the Ring Size of Epothilones: Total Synthesis of 14-, 15-, 17-, and 18 Epothilones A". Angewandte Chemie International Edition in English. 37 (1–2): 81–84. doi:10.1002/(sici)1521-3773(19980202)37:1/2<81::aid-anie81>3.0.co;2-c.
- ^ Batey, Robert A.; Quach Tan D.; Shen Ming; Thadani, Avinash N.; Smil, David V.; Li Sze-Wan; MacKay, D. Bruce (2002). "Organoboron compounds as mild nucleophiles in Lewis acid- and transition metal-catalyzed C–C bond-forming reactions" (PDF). Pure Appl. Chem. 74 (1): 43–55. doi:10.1351/pac200274010043. S2CID 49223984.
- ^ a b Midland, M. M.; Brown, H. C. J. Org. Chem. 1975, 40, 2845.
- ^ Brown, H. C.; Rathke, M. W.; Rogic, M. M. J. Am. Chem. Soc. 1968, 90, 5038.
- ^ Negishi, E.-i.; Lew, G.; Yoshida T. Chem. Commun. 1973, 874.
- ^ Miyaura Norio; Suzuki Akira (1995). "Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds". Chemical Reviews. 95 (7): 2457–2483. CiteSeerX 10.1.1.735.7660. doi:10.1021/cr00039a007. S2CID 53050782.
- ^ Rathke, M. W.; Brown, H. C. J. Am. Chem. Soc. 1967, 89, 2740.
- ^ Negishi E.-I.; Abramovitch, A.; Merrill, R. E. Chem. Commun. 1975, 138.
- ^ Barth, Rolf F.; Mi, Peng; Yang, Weilian (December 2018). "Boron delivery agents for neutron capture therapy of cancer". Cancer Communications. 38 (1): 35. doi:10.1186/s40880-018-0299-7. ISSN 2523-3548. PMC 6006782. PMID 29914561.

![{\displaystyle {\ce {R1-BY2{}+R2-X->[{} \atop {\underset {\text{catalyst}}{\ce {Pd}}}][{\text{Base}}]R1-R2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4242d81e9b075c35d4c3fb6635b7ecd3bb4a865b)