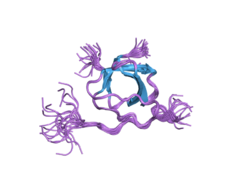
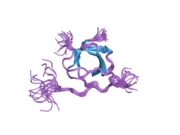LYN
Tyrosine-protein kinase Lyn is a protein that in humans is encoded by the LYN gene.[5]
Lyn is a member of the Src family of protein tyrosine kinases, which is mainly expressed in hematopoietic cells,[6] in neural tissues[7] liver, and adipose tissue.[8] In various hematopoietic cells, Lyn has emerged as a key enzyme involved in the regulation of cell activation. In these cells, a small amount of LYN is associated with cell surface receptor proteins, including the B cell antigen receptor (BCR),[9][10] CD40,[11] or CD19.[12] The abbreviation Lyn is derived from Lck/Yes novel tyrosine kinase, Lck and Yes also being members of the Src kinase family.
Function
[edit]Lyn has been described to have an inhibitory role in myeloid lineage proliferation.[13] Following engagement of the B cell receptors, Lyn undergoes rapid phosphorylation and activation. LYN activation triggers a cascade of signaling events mediated by Lyn phosphorylation of tyrosine residues within the immunoreceptor tyrosine-based activation motifs (ITAM) of the receptor proteins, and subsequent recruitment and activation of other kinases including Syk, phosholipase Cγ2 (PLCγ2) and phosphatidyl inositol-3 kinase.[12][14] These kinases provide activation signals, which play critical roles in proliferation, Ca2+ mobilization and cell differentiation. Lyn plays an essential role in the transmission of inhibitory signals through phosphorylation of tyrosine residues within the immunoreceptor tyrosine-based inhibitory motifs (ITIM) in regulatory proteins such as CD22, PIR-B and FCγRIIb1. Their ITIM phosphorylation subsequently leads to recruitment and activation of phosphatases such as SHIP-1 and SHP-1,[15][16][17][18][19] which further downmodulate signaling pathways, attenuate cell activation and can mediate tolerance. In B cells, Lyn sets the threshold of cell signaling and maintains the balance between activation and inhibition. Lyn thus functions as a rheostat that modulates signaling rather than as a binary on-off switch.[20][21][22] HSP90 inhibitor NVP-BEP800 has been described to affect stability of LYN kinase and growth of B-cell acute lymphoblastic leukemias through inhibition of the NF-kappaB signaling. [23]
LYN is reported to be a key signal mediator for estrogen-dependent suppression of human osteoclast differentiation, survival, and function.[24] Lyn has also been implicated to have a role in the insulin signaling pathway. Activated Lyn phosphorylates insulin receptor substrate 1 (IRS1). This phosphorylation of IRS1 leads to an increase in translocation of Glut-4 to the cell membrane and increased glucose utilization.[25] In turn, activation of the insulin receptor has been shown to increase autophosphorylation of Lyn suggesting a possible feedback loop.[26] The insulin secretagogue, glimepiride (Amaryl®) activates Lyn in adipocytes via the disruption of lipid rafts.[27] This indirect Lyn activation may modulate the extrapancreatic glycemic control activity of glimepiride.[27][28] Tolimidone (MLR-1023) is a small molecule allosteric activator of lyn kinase with an EC50 of 63 nM[29][30] that is currently under Phase 2a investigation for Type II diabetes.[31] In June, 2016, the sponsor of these studies, Melior Discovery, announced positive results from their Phase 2a study with tolimidone in diabetic patients,[32][33] and the continuation of additional clinical studies.[34]
Lyn has been shown to protect against hepatocellular apoptosis and promote liver regeneration through the preservation of hepatocellular mitochondrial integrity.[35]
Several investigators have shown the role of lyn kinase in different aspects of pulmonary function. Lyn activation in pulmonary epithelium has been shown to be important in improving pulmonary barrier integrity and to reduce edema.[36][37] Additional evidence suggest that lyn activation in alveolar phagocytes improves phagocytosis of bacteria and reduces pulmonary infection. [38][39] Finally, other research has found that lyn activation reduces pulmonary hypersecretion of mucus. [40]
Pathology
[edit]Much of the current knowledge about Lyn has emerged from studies of genetically manipulated mice. Lyn deficient mice display a phenotype that includes splenomegaly, a dramatic increase in numbers of myeloid progenitors and monocyte/macrophage tumors. Biochemical analysis of cells from these mutants revealed that Lyn is essential in establishing ITIM-dependent inhibitory signaling and for activation of specific protein tyrosine phosphatases within myeloid cells.[13]
Mice that expressed a hyperactive Lyn allele were tumor free and displayed no propensity toward hematological malignancy. These mice have reduced numbers of conventional B lymphocytes, down-regulated surface immunoglobulin M and costimulatory molecules, and elevated numbers of B1a B cells. With age these animals developed a glomerulonephritis phenotype associated with a 30% reduction in life expectancy.[41]
Interactions
[edit]LYN has been shown to interact with:
See also
[edit]References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000254087 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000042228 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Yamanashi Y, Fukushige S, Semba K, Sukegawa J, Miyajima N, Matsubara K, Yamamoto T, Toyoshima K (Jan 1987). "The yes-related cellular gene lyn encodes a possible tyrosine kinase similar to p56lck". Molecular and Cellular Biology. 7 (1): 237–43. doi:10.1128/MCB.7.1.237. PMC 365062. PMID 3561390.
- ^ Yamanashi Y, Mori S, Yoshida M, Kishimoto T, Inoue K, Yamamoto T, Toyoshima K (Sep 1989). "Selective expression of a protein-tyrosine kinase, p56lyn, in hematopoietic cells and association with production of human T-cell lymphotropic virus type I". Proceedings of the National Academy of Sciences of the United States of America. 86 (17): 6538–42. Bibcode:1989PNAS...86.6538Y. doi:10.1073/pnas.86.17.6538. PMC 297879. PMID 2505253.
- ^ Umemori H, Wanaka A, Kato H, Takeuchi M, Tohyama M, Yamamoto T (Dec 1992). "Specific expressions of Fyn and Lyn, lymphocyte antigen receptor-associated tyrosine kinases, in the central nervous system". Brain Research. Molecular Brain Research. 16 (3–4): 303–10. doi:10.1016/0169-328X(92)90239-8. PMID 1337939.
- ^ Yamada E, Pessin JE, Kurland IJ, Schwartz GJ, Bastie CC (Feb 2010). "Fyn-dependent regulation of energy expenditure and body weight is mediated by tyrosine phosphorylation of LKB1". Cell Metabolism. 11 (2): 113–124. doi:10.1016/j.cmet.2009.12.010. PMC 2830006. PMID 20142099.
- ^ Yamamoto T, Yamanashi Y, Toyoshima K (Apr 1993). "Association of Src-family kinase Lyn with B-cell antigen receptor". Immunological Reviews. 132: 187–206. doi:10.1111/j.1600-065X.1993.tb00843.x. PMID 8349296. S2CID 10782326.
- ^ Campbell MA, Sefton BM (May 1992). "Association between B-lymphocyte membrane immunoglobulin and multiple members of the Src family of protein tyrosine kinases". Molecular and Cellular Biology. 12 (5): 2315–21. doi:10.1128/MCB.12.5.2315. PMC 364403. PMID 1569953.
- ^ Ren CL, Morio T, Fu SM, Geha RS (Feb 1994). "Signal transduction via CD40 involves activation of lyn kinase and phosphatidylinositol-3-kinase, and phosphorylation of phospholipase C gamma 2". The Journal of Experimental Medicine. 179 (2): 673–80. doi:10.1084/jem.179.2.673. PMC 2191357. PMID 7507510.
- ^ a b Campbell 1999
- ^ a b Harder KW, Parsons LM, Armes J, Evans N, Kountouri N, Clark R, Quilici C, Grail D, Hodgson GS, Dunn AR, Hibbs ML (Oct 2001). "Gain- and loss-of-function Lyn mutant mice define a critical inhibitory role for Lyn in the myeloid lineage". Immunity. 15 (4): 603–615. doi:10.1016/S1074-7613(01)00208-4. PMID 11672542.
- ^ Yamanashi Y, Fukui Y, Wongsasant B, Kinoshita Y, Ichimori Y, Toyoshima K, Yamamoto T (Feb 1992). "Activation of Src-like protein-tyrosine kinase Lyn and its association with phosphatidylinositol 3-kinase upon B-cell antigen receptor-mediated signaling". Proceedings of the National Academy of Sciences of the United States of America. 89 (3): 1118–22. Bibcode:1992PNAS...89.1118Y. doi:10.1073/pnas.89.3.1118. PMC 48397. PMID 1371009.
- ^ Cornall RJ, Cyster JG, Hibbs ML, Dunn AR, Otipoby KL, Clark EA, Goodnow CC (Apr 1998). "Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection". Immunity. 8 (4): 497–508. doi:10.1016/S1074-7613(00)80554-3. PMID 9586639.
- ^ Smith KG, Tarlinton DM, Doody GM, Hibbs ML, Fearon DT (Mar 1998). "Inhibition of the B cell by CD22: a requirement for Lyn". The Journal of Experimental Medicine. 187 (5): 807–11. doi:10.1084/jem.187.5.807. PMC 2212179. PMID 9480991.
- ^ Chan VW, Lowell CA, DeFranco AL (May 1998). "Defective negative regulation of antigen receptor signaling in Lyn-deficient B lymphocytes". Current Biology. 8 (10): 545–53. Bibcode:1998CBio....8..545C. doi:10.1016/S0960-9822(98)70223-4. PMID 9601638. S2CID 12195731.
- ^ Nishizumi H, Horikawa K, Mlinaric-Rascan I, Yamamoto T (Apr 1998). "A double-edged kinase Lyn: a positive and negative regulator for antigen receptor-mediated signals". The Journal of Experimental Medicine. 187 (8): 1343–8. doi:10.1084/jem.187.8.1343. PMC 2212230. PMID 9547345.
- ^ Maeda A, Scharenberg AM, Tsukada S, Bolen JB, Kinet JP, Kurosaki T (Apr 1999). "Paired immunoglobulin-like receptor B (PIR-B) inhibits BCR-induced activation of Syk and Btk by SHP-1". Oncogene. 18 (14): 2291–7. doi:10.1038/sj.onc.1202552. PMID 10327049.
- ^ Lowell CA (Jul 2004). "Src-family kinases: rheostats of immune cell signaling". Molecular Immunology. 41 (6–7): 631–43. doi:10.1016/j.molimm.2004.04.010. PMID 15220000.
- ^ Saijo K, Schmedt C, Su IH, Karasuyama H, Lowell CA, Reth M, Adachi T, Patke A, Santana A, Tarakhovsky A (Mar 2003). "Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development". Nature Immunology. 4 (3): 274–9. doi:10.1038/ni893. PMID 12563261. S2CID 32559368.
- ^ Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM (Jan 2005). "Lyn tyrosine kinase: accentuating the positive and the negative". Immunity. 22 (1): 9–18. doi:10.1016/j.immuni.2004.12.004. PMID 15664155.
- ^ Mshaik R, Simonet J, Georgievski A, Jamal L, Bechoua S, Ballerini P, Bellaye PS, Mlamla Z, Pais de Barros JP, Geissler A, Francin PJ, Girodon F, Garrido C, Quéré R (March 2021). "HSP90 inhibitor NVP-BEP800 affects stability of SRC kinases and growth of T-cell and B-cell acute lymphoblastic leukemia". Blood Cancer J. 3 (11): 61. doi:10.1038/s41408-021-00450-2. PMC 7973815. PMID 33737511.
- ^ Gavali S, Gupta MK, Daswani B, Wani MR, Sirdeshmukh R, Khatkhatay MI (March 2019). "LYN, a key mediator in estrogen-dependent suppression of osteoclast differentiation, survival, and function". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1865 (3): 547–557. doi:10.1016/j.bbadis.2018.12.016. PMID 30579930.
- ^ Müller G, Wied S, Frick W (Jul 2000). "Cross talk of pp125(FAK) and pp59(Lyn) non-receptor tyrosine kinases to insulin-mimetic signaling in adipocytes". Molecular and Cellular Biology. 20 (13): 4708–4723. doi:10.1128/mcb.20.13.4708-4723.2000. PMC 85892. PMID 10848597.
- ^ Anderwald C, Müller G, Koca G, Fürnsinn C, Waldhäusl W, Roden M (Jul 2002). "Short-term leptin-dependent inhibition of hepatic gluconeogenesis is mediated by insulin receptor substrate-2". Molecular Endocrinology. 16 (7): 1612–1628. doi:10.1210/mend.16.7.0867. PMID 12089355.
- ^ a b Müller G (Nov 2000). "The molecular mechanism of the insulin-mimetic/sensitizing activity of the antidiabetic sulfonylurea drug Amaryl". Molecular Medicine. 6 (11): 907–933. doi:10.1007/BF03401827. PMC 1949923. PMID 11147570.
- ^ Müller G, Schulz A, Wied S, Frick W (Mar 2005). "Regulation of lipid raft proteins by glimepiride- and insulin-induced glycosylphosphatidylinositol-specific phospholipase C in rat adipocytes". Biochemical Pharmacology. 69 (5): 761–780. doi:10.1016/j.bcp.2004.11.014. PMID 15710354.
- ^ Ochman AR, Lipinski CA, Handler JA, Reaume AG, Saporito MS (2012). "The Lyn kinase activator MLR-123 is a novel insulin receptor potentiator that elicits a rapid-onset and durable improvement in glucose homeostasis in animal models of type 2 diabetes". J Pharmacol Exp Ther. 342 (1): 23–32. doi:10.1124/jpet.112.192187. PMID 22431203. S2CID 7288053.[permanent dead link]
- ^ Saporito MS, Ochman AR, Lipinski CA, Handler JA, Reaume AG (2011). "MLR-1023 is potent and selective allosteric activator of Lyn kinase in vitro that improves glucose tolerance in vivo". Journal of Pharmacology and Experimental Therapeutics. 342 (1): 15–22. doi:10.1124/jpet.112.192096. PMID 22473614. S2CID 26419896.
- ^ "Melior Pharmaceuticals Initiates Phase 2 Study with MLR-1023 for Type 2 Diabetes". Business Wire. 3 March 2015. Retrieved March 3, 2015.
- ^ "Melior Pharmaceuticals Announces Positive Phase 2A Results in Type 2 Diabetes Study". www.businesswire.com. 13 June 2016. Retrieved 2017-08-04.
- ^ Lee MK, Kim SG, Watkins E, Moon MK, Rhee SY, Frias JP, Chung CH, Lee SH, Block B, Cha BS, Park HK, Kim BJ, Greenway F (May 2020). "A Novel Non-PPARgamma Insulin Sensitizer: MLR-1023 Clinical Proof-of-concept in Type 2 Diabetes Mellitus". J. Diabetes Complications. 34 (5): 107555. doi:10.1016/j.jdiacomp.2020.107555. PMID 32019723. S2CID 211036334.
- ^ "Melior Discovery website press releases". Archived from the original on 2018-05-17. Retrieved 2018-06-11.
- ^ Gringeri E, Carraro A, Tibaldi E, D'Amico FE, Mancon M, Toninello A, Pagano MA, Vio C, Cillo U, Brunati AM (December 2009). "Lyn-mediated mitochondrial tyrosine phosphorylation is required to preserve mitochondrial integrity in early liver regeneration" (PDF). The Biochemical Journal. 425 (2): 401–12. doi:10.1042/BJ20090902. PMID 19832701.
- ^ Jan J, Zhang G, Welch EJ, Liang Y, Fu J, Vogel SM, Lowell CA, Du X, Cheresh DA, Malik AB, Li Z (Dec 2013). "A critical role for Lyn kinase in strengthening endothelial integrity and barrier function". Blood. 122 (25): 4140–4149. doi:10.1182/blood-2013-03-491423. PMC 3862279. PMID 24108461.
- ^ Corey SJ, Day RM (Dec 2013). "Shockingly: the loss of Lyn leads to leakiness". Blood. 122 (25): 4009–4010. doi:10.1182/blood-2013-10-533158. PMC 3862277. PMID 24335031.
- ^ Li X, He S, Zhou X, Ye Y, Tan S, Zhang S, Li R, Yu M, Jundt MC, Hidebrand A, Wang Y, Li G, Huang C, Wu M (Jan 2016). "Lyn delivers bacteria to lysosomes for eradication through TLR2-initiated autophagy related phagocytosis". PLOS Pathogens. 12 (1): e1005363. doi:10.1371/journal.ppat.1005363. PMC 4703367. PMID 26735693.
- ^ Li X, Zhou X, Ye Y, Li Y, Li J, Privratsky B, Wu E, Gao H, Huang C, Wu M (2012). "Lyn regulates inflammatory responses in Klebsiella pneumoniae infection via the p38/NF-κB pathway". Eur. J. Immunol. 44 (3): 763–773. doi:10.1002/eji.201343972. PMC 4103995. PMID 24338528.
- ^ Wang X, Li Y, Wang X, Zhang Y, Liu Z, Zhong N, Wu M, Li G (Feb 2016). "Lyn regulates mucus secretion and MUC5AC via the STAT6 signaling pathway during allergic airway inflammation". Sci Rep. 7: 42675. doi:10.1038/srep42675. PMC 5312001. PMID 28205598.
- ^ Hibbs ML, Harder KW, Armes J, Kountouri N, Quilici C, Casagranda F, Dunn AR, Tarlinton DM (Dec 2002). "Sustained activation of Lyn tyrosine kinase in vivo leads to autoimmunity". The Journal of Experimental Medicine. 196 (12): 1593–604. doi:10.1084/jem.20020515. PMC 2196073. PMID 12486102.
- ^ a b Manié SN, Beck AR, Astier A, Law SF, Canty T, Hirai H, Druker BJ, Avraham H, Haghayeghi N, Sattler M, Salgia R, Griffin JD, Golemis EA, Freedman AS (Feb 1997). "Involvement of p130(Cas) and p105(HEF1), a novel Cas-like docking protein, in a cytoskeleton-dependent signaling pathway initiated by ligation of integrin or antigen receptor on human B cells". The Journal of Biological Chemistry. 272 (7): 4230–6. doi:10.1074/jbc.272.7.4230. hdl:20.500.12613/9177. PMID 9020138.
- ^ Qiu W, Cobb RR, Scholz W (May 1998). "Inhibition of p130cas tyrosine phosphorylation by calyculin A". Journal of Leukocyte Biology. 63 (5): 631–5. doi:10.1002/jlb.63.5.631. PMID 9581808. S2CID 11177730.
- ^ a b Liang X, Wisniewski D, Strife A, Clarkson B, Resh MD (Apr 2002). "Phosphatidylinositol 3-kinase and Src family kinases are required for phosphorylation and membrane recruitment of Dok-1 in c-Kit signaling". The Journal of Biological Chemistry. 277 (16): 13732–8. doi:10.1074/jbc.M200277200. PMID 11825908.
- ^ Linnekin D, DeBerry CS, Mou S (Oct 1997). "Lyn associates with the juxtamembrane region of c-Kit and is activated by stem cell factor in hematopoietic cell lines and normal progenitor cells". The Journal of Biological Chemistry. 272 (43): 27450–5. doi:10.1074/jbc.272.43.27450. PMID 9341198.
- ^ Poe JC, Fujimoto M, Jansen PJ, Miller AS, Tedder TF (Jun 2000). "CD22 forms a quaternary complex with SHIP, Grb2, and Shc. A pathway for regulation of B lymphocyte antigen receptor-induced calcium flux". The Journal of Biological Chemistry. 275 (23): 17420–7. doi:10.1074/jbc.M001892200. PMID 10748054.
- ^ Greer SF, Justement LB (May 1999). "CD45 regulates tyrosine phosphorylation of CD22 and its association with the protein tyrosine phosphatase SHP-1". Journal of Immunology. 162 (9): 5278–86. doi:10.4049/jimmunol.162.9.5278. PMID 10228003.
- ^ Kharbanda S, Yuan ZM, Rubin E, Weichselbaum R, Kufe D (Aug 1994). "Activation of Src-like p56/p53lyn tyrosine kinase by ionizing radiation". The Journal of Biological Chemistry. 269 (32): 20739–43. doi:10.1016/S0021-9258(17)32054-9. PMID 8051175.
- ^ Pathan NI, Geahlen RL, Harrison ML (Nov 1996). "The protein-tyrosine kinase Lck associates with and is phosphorylated by Cdc2". The Journal of Biological Chemistry. 271 (44): 27517–23. doi:10.1074/jbc.271.44.27517. PMID 8910336.
- ^ van Dijk TB, van Den Akker E, Amelsvoort MP, Mano H, Löwenberg B, von Lindern M (Nov 2000). "Stem cell factor induces phosphatidylinositol 3'-kinase-dependent Lyn/Tec/Dok-1 complex formation in hematopoietic cells". Blood. 96 (10): 3406–13. doi:10.1182/blood.V96.10.3406. hdl:1765/9530. PMID 11071635.
- ^ Chin H, Arai A, Wakao H, Kamiyama R, Miyasaka N, Miura O (May 1998). "Lyn physically associates with the erythropoietin receptor and may play a role in activation of the Stat5 pathway". Blood. 91 (10): 3734–45. doi:10.1182/blood.V91.10.3734. PMID 9573010.
- ^ Suzuki-Inoue K, Tulasne D, Shen Y, Bori-Sanz T, Inoue O, Jung SM, Moroi M, Andrews RK, Berndt MC, Watson SP (Jun 2002). "Association of Fyn and Lyn with the proline-rich domain of glycoprotein VI regulates intracellular signaling". The Journal of Biological Chemistry. 277 (24): 21561–6. doi:10.1074/jbc.M201012200. PMID 11943772.
- ^ Baran CP, Tridandapani S, Helgason CD, Humphries RK, Krystal G, Marsh CB (Oct 2003). "The inositol 5'-phosphatase SHIP-1 and the Src kinase Lyn negatively regulate macrophage colony-stimulating factor-induced Akt activity". The Journal of Biological Chemistry. 278 (40): 38628–36. doi:10.1074/jbc.M305021200. PMID 12882960.
- ^ Müller G, Wied S, Frick W (Jul 2000). "Cross talk of pp125(FAK) and pp59(Lyn) non-receptor tyrosine kinases to insulin-mimetic signaling in adipocytes". Molecular and Cellular Biology. 20 (13): 4708–23. doi:10.1128/mcb.20.13.4708-4723.2000. PMC 85892. PMID 10848597.
- ^ Gross BS, Lee JR, Clements JL, Turner M, Tybulewicz VL, Findell PR, Koretzky GA, Watson SP (Feb 1999). "Tyrosine phosphorylation of SLP-76 is downstream of Syk following stimulation of the collagen receptor in platelets". The Journal of Biological Chemistry. 274 (9): 5963–71. doi:10.1074/jbc.274.9.5963. PMID 10026222.
- ^ Durum SK, Aiello FB (2003). "Interleukin-7 induces MUC1". Cancer Biology & Therapy. 2 (2): 194–5. doi:10.4161/cbt.2.2.351. PMID 12750562.
- ^ Pleiman CM, Clark MR, Gauen LK, Winitz S, Coggeshall KM, Johnson GL, Shaw AS, Cambier JC (Sep 1993). "Mapping of sites on the Src family protein tyrosine kinases p55blk, p59fyn, and p56lyn which interact with the effector molecules phospholipase C-gamma 2, microtubule-associated protein kinase, GTPase-activating protein, and phosphatidylinositol 3-kinase". Molecular and Cellular Biology. 13 (9): 5877–87. doi:10.1128/MCB.13.9.5877. PMC 360336. PMID 8395016.
- ^ Guo B, Kato RM, Garcia-Lloret M, Wahl MI, Rawlings DJ (Aug 2000). "Engagement of the human pre-B cell receptor generates a lipid raft-dependent calcium signaling complex". Immunity. 13 (2): 243–53. doi:10.1016/s1074-7613(00)00024-8. PMID 10981967.
- ^ Grishin AV, Azhipa O, Semenov I, Corey SJ (Aug 2001). "Interaction between growth arrest-DNA damage protein 34 and Src kinase Lyn negatively regulates genotoxic apoptosis". Proceedings of the National Academy of Sciences of the United States of America. 98 (18): 10172–7. Bibcode:2001PNAS...9810172G. doi:10.1073/pnas.191130798. PMC 56934. PMID 11517336.
- ^ Brown VK, Ogle EW, Burkhardt AL, Rowley RB, Bolen JB, Justement LB (Jun 1994). "Multiple components of the B cell antigen receptor complex associate with the protein tyrosine phosphatase, CD45". The Journal of Biological Chemistry. 269 (25): 17238–44. doi:10.1016/S0021-9258(17)32545-0. PMID 7516335.
- ^ Sidorenko SP, Law CL, Chandran KA, Clark EA (Jan 1995). "Human spleen tyrosine kinase p72Syk associates with the Src-family kinase p53/56Lyn and a 120-kDa phosphoprotein". Proceedings of the National Academy of Sciences of the United States of America. 92 (2): 359–63. Bibcode:1995PNAS...92..359S. doi:10.1073/pnas.92.2.359. PMC 42739. PMID 7831290.
- ^ Xu H, Zhao H, Tian W, Yoshida K, Roullet JB, Cohen DM (Mar 2003). "Regulation of a transient receptor potential (TRP) channel by tyrosine phosphorylation. SRC family kinase-dependent tyrosine phosphorylation of TRPV4 on TYR-253 mediates its response to hypotonic stress". The Journal of Biological Chemistry. 278 (13): 11520–7. doi:10.1074/jbc.M211061200. PMID 12538589.
- ^ Cen O, Gorska MM, Stafford SJ, Sur S, Alam R (Mar 2003). "Identification of UNC119 as a novel activator of SRC-type tyrosine kinases". The Journal of Biological Chemistry. 278 (10): 8837–45. doi:10.1074/jbc.M208261200. PMID 12496276.
Further reading
[edit]- Jouvin MH, Numerof RP, Kinet JP (Feb 1995). "Signal transduction through the conserved motifs of the high affinity IgE receptor Fc epsilon RI". Seminars in Immunology. 7 (1): 29–35. doi:10.1016/1044-5323(95)90005-5. PMID 7612892.
- Hibbs ML, Dunn AR (Mar 1997). "Lyn, a src-like tyrosine kinase". The International Journal of Biochemistry & Cell Biology. 29 (3): 397–400. doi:10.1016/S1357-2725(96)00104-5. PMID 9202419.
- Blasioli J, Goodnow CC (2002). Lyn/CD22/SHP-1 and Their Importance in Autoimmunity. Vol. 5. pp. 151–60. doi:10.1159/000060551. ISBN 978-3-8055-7308-5. PMID 11826756.
{{cite book}}:|journal=ignored (help) - Greenway AL, Holloway G, McPhee DA, Ellis P, Cornall A, Lidman M (Apr 2003). "HIV-1 Nef control of cell signalling molecules: multiple strategies to promote virus replication". Journal of Biosciences. 28 (3): 323–35. doi:10.1007/BF02970151. PMID 12734410. S2CID 33749514.
- Tolstrup M, Ostergaard L, Laursen AL, Pedersen SF, Duch M (Apr 2004). "HIV/SIV escape from immune surveillance: focus on Nef". Current HIV Research. 2 (2): 141–51. doi:10.2174/1570162043484924. PMID 15078178.
- Joseph AM, Kumar M, Mitra D (Jan 2005). "Nef: "necessary and enforcing factor" in HIV infection". Current HIV Research. 3 (1): 87–94. doi:10.2174/1570162052773013. PMID 15638726.
- Stove V, Verhasselt B (Jan 2006). "Modelling thymic HIV-1 Nef effects". Current HIV Research. 4 (1): 57–64. doi:10.2174/157016206775197583. PMID 16454711.