Diprophylline
Appearance
From Wikipedia, the free encyclopedia
(Redirected from Lufyllin)
Chemical compound
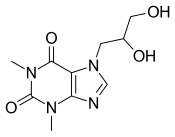 | |
| Clinical data | |
|---|---|
| Trade names | Lufyllin |
| Other names | 7-(2,3-Dihydroxy-propyl)theophylline |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a682494 |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.843 |
| Chemical and physical data | |
| Formula | C10H14N4O4 |
| Molar mass | 254.246 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Diprophylline (INN)[1] or dyphylline (USAN) (trade names Dilor, Lufyllin) is a xanthine derivative with bronchodilator and vasodilator effects. It is used in the treatment of respiratory disorders like asthma, cardiac dyspnea, and bronchitis. It acts as an adenosine receptor antagonist and phosphodiesterase inhibitor.[2][3]
See also
[edit]References
[edit]- ^ "International Non-Proprietary Names. Recommended International Non-Proprietary Names (Rec. I.N.N.): List 1" (PDF). World Health Organization. 1955. p. 188.
- ^ Schwabe U, Ukena D, Lohse MJ (September 1985). "Xanthine derivatives as antagonists at A1 and A2 adenosine receptors". Naunyn-Schmiedeberg's Archives of Pharmacology. 330 (3): 212–21. doi:10.1007/bf00572436. PMID 2997628. S2CID 12190457.
- ^ Iancu L, Shneur A, Cohen H (1979). "Trials with xanthine derivatives in systemic treatment of psoriasis". Dermatologica. 159 (1): 55–61. doi:10.1159/000250562. PMID 225216.
| Receptor (ligands) |
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (blockers) |
| ||||||||||
| Enzyme (inhibitors) |
| ||||||||||
| Others | |||||||||||
See also: Receptor/signaling modulators | |||||||||||
| Adamantanes | |
|---|---|
| Adenosine antagonists | |
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cathinones |
|
| Cholinergics |
|
| Convulsants | |
| Eugeroics | |
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines | |
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines | |
| Others |
|
| PDE1 | |
|---|---|
| PDE2 | |
| PDE3 | |
| PDE4 |
|
| PDE5 | |
| PDE7 | |
| PDE9 | |
| PDE10 | |
| PDE11 | |
| Non-selective | |
| Unsorted | |
See also: Receptor/signaling modulators | |
This drug article relating to the respiratory system is a stub. You can help Wikipedia by expanding it. |
Retrieved from "http://en.wiki.x.io/w/index.php?title=Diprophylline&oldid=1188048844"
