Amniote
This article needs additional citations for verification. (August 2022) |
| Amniotes Temporal range: (Possible Mississippian record)
| |
|---|---|

| |
| From top to bottom and left to right, examples of amniotes: Edaphosaurus, red fox (two synapsids), king cobra and a white-headed buffalo weaver (two sauropsids). | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Superclass: | Tetrapoda |
| Clade: | Reptiliomorpha |
| Clade: | Amniota Haeckel, 1866 |
| Clades | |
Amniotes are tetrapod vertebrate animals belonging to the clade Amniota, a large group that comprises the vast majority of living terrestrial and semiaquatic vertebrates. Amniotes evolved from amphibian ancestors during the Carboniferous period and further diverged into two groups, namely the sauropsids (including all reptiles and birds) and synapsids (including mammals and extinct ancestors like "pelycosaurs" and therapsids), an event that marks the appearance of Amniota, according to the definition[4] established under the PhyloCode.[5] This basal divergence within Amniota has been dated by molecular studies at 310–329 Ma[6] or 312–330 Ma,[7] but the presence of Hylonomus at Joggins implies a minimal age of about 317 Ma.[8] A fossilized birth-death process study of early amniotes suggested an age of 322–340 Ma.[9] Amniotes are distinguished from the other living tetrapod clade — the non-amniote lissamphibians (frogs/toads, salamanders, newts and caecilians) — by the development of three extraembryonic membranes (amnion for embryonic protection, chorion for gas exchange, and allantois for metabolic waste disposal or storage), thicker and keratinized skin, and costal respiration (breathing by expanding/constricting the rib cage).[10][11][12][13] Additional unique features are the presence of adrenocortical and chromaffin tissues as a discrete pair of glands[14]: 600 near their kidneys, which are more complex,[14]: 552 the presence of an astragalus for better extremity range of motion,[15] the diminished role of skin breathing, and the complete loss of metamorphosis, gills, and lateral lines.[14]: 694
All three main amniote features listed above, namely the presence of an amniotic buffer, water-impermeable cutes and a robust air-breathing respiratory system, are very important for living on land as true terrestrial animals — the ability to survive and procreate in locations away from water bodies, better homeostasis in drier environments, and more efficient non-aquatic gas exchange to power terrestrial locomotions, although they might still require regular access to drinking water for rehydration like the semiaquatic amphibians do. Because the amnion and the fluid it secretes shields the embryo from environmental fluctuations, amniotes can reproduce on dry land by either laying shelled eggs (reptiles, birds and monotremes) or nurturing fertilized eggs within the mother (marsupial and placental mammals), unlike anamniotes (fish and amphibians) that have to spawn in or closely adjacent to aquatic environments.
Among the earliest known crown group amniotes, the oldest known sauropsid is Hylonomus and the oldest known synapsid is Asaphestera, both of which are from Nova Scotia during the Bashkirian age of the Late Carboniferous around 318 million years ago.[1][16] Basal amniotes resembled small lizards and evolved from semiaquatic reptiliomorphs during the Carboniferous period.[17] After the Carboniferous rainforest collapse, amniotes spread around Earth's land and became the dominant land vertebrates,[17] and soon diverged into the synapsids and sauropsids, whose lineages both still persist today. Older sources, particularly before the 20th century, may refer to amniotes as "higher vertebrates" and anamniotes as "lower vertebrates", based on the antiquated idea of the evolutionary great chain of being.
Etymology
[edit]The term amniote comes from the amnion, which derives from Greek ἀμνίον (amnion), which denoted the membrane that surrounds a fetus. The term originally described a bowl in which the blood of sacrificed animals was caught, and derived from ἀμνός (amnos), meaning "lamb".[18]
Description
[edit]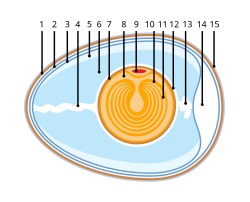
- Eggshell
- Outer membrane
- Inner membrane
- Chalaza
- Exterior albumen (outer thin albumen)
- Middle albumen (inner thick albumen)
- Vitelline membrane
- Nucleus of Pander
- Germinal disk (blastoderm)
- Yellow yolk
- White yolk
- Internal albumen
- Chalaza
- Air cell
- Cuticula
Zoologists characterize amniotes in part by embryonic development that includes the formation of several extensive membranes, the amnion, chorion, and allantois. Amniotes develop directly into a (typically) terrestrial form with limbs and a thick stratified epithelium (rather than first entering a feeding larval tadpole stage followed by metamorphosis, as amphibians do). In amniotes, the transition from a two-layered periderm to a cornified epithelium is triggered by thyroid hormone during embryonic development, rather than by metamorphosis.[19] The unique embryonic features of amniotes may reflect specializations for eggs to survive drier environments; or the increase in size and yolk content of eggs may have permitted, and coevolved with, direct development of the embryo to a large size.
Adaptation for terrestrial living
[edit]Features of amniotes evolved for survival on land include a sturdy but porous leathery or hard eggshell and an allantois that facilitates respiration while providing a reservoir for disposal of wastes. Their kidneys (metanephros) and large intestines are also well-suited to water retention. Most mammals do not lay eggs, but corresponding structures develop inside the placenta.
The ancestors of true amniotes, such as Casineria kiddi, which lived about 340 million years ago, evolved from amphibian reptiliomorphs and resembled small lizards. At the late Devonian mass extinction (360 million years ago), all known tetrapods were essentially aquatic and fish-like. Because the reptiliomorphs were already established 20 million years later when all their fishlike relatives were extinct, it appears they separated from the other tetrapods somewhere during Romer's gap, when the adult tetrapods became fully terrestrial (some forms would later become secondarily aquatic).[20] The modest-sized ancestors of the amniotes laid their eggs in moist places, such as depressions under fallen logs or other suitable places in the Carboniferous swamps and forests; and dry conditions probably do not account for the emergence of the soft shell.[21] Indeed, many modern-day amniotes require moisture to keep their eggs from desiccating.[22] Although some modern amphibians lay eggs on land, all amphibians lack advanced traits like an amnion.
The amniotic egg formed through a series of evolutionary steps. After internal fertilization and the habit of laying eggs in terrestrial environments became a reproduction strategy amongst the amniote ancestors, the next major breakthrough appears to have involved a gradual replacement of the gelatinous coating covering the amphibian egg with a fibrous shell membrane. This allowed the egg to increase both its size and in the rate of gas exchange, permitting a larger, metabolically more active embryo to reach full development before hatching. Further developments, like extraembryonic membranes (amnion, chorion, and allantois) and a calcified shell, were not essential and probably evolved later.[23] It has been suggested that shelled terrestrial eggs without extraembryonic membranes could still not have been more than about 1 cm (0.4-inch) in diameter because of diffusion problems, like the inability to get rid of carbon dioxide if the egg was larger. The combination of small eggs and the absence of a larval stage, where posthatching growth occurs in anamniotic tetrapods before turning into juveniles, would limit the size of the adults. This is supported by the fact that extant squamate species that lay eggs less than 1 cm in diameter have adults whose snout-vent length is less than 10 cm. The only way for the eggs to increase in size would be to develop new internal structures specialized for respiration and for waste products. As this happened, it would also affect how much the juveniles could grow before they reached adulthood.[24]
A similar pattern can be seen in modern amphibians. Frogs that have evolved terrestrial reproduction and direct development have both smaller adults and fewer and larger eggs compared to their relatives that still reproduce in water.[25]
The egg membranes
[edit]Fish and amphibian eggs have only one inner membrane, the embryonic membrane. Evolution of the amniote egg required increased exchange of gases and wastes between the embryo and the atmosphere. Structures to permit these traits allowed further adaption that increased the feasible size of amniote eggs and enabled breeding in progressively drier habitats. The increased size of eggs permitted increase in size of offspring and consequently of adults. Further growth for the latter, however, was limited by their position in the terrestrial food-chain, which was restricted to level three and below, with only invertebrates occupying level two. Amniotes would eventually experience adaptive radiations when some species evolved the ability to digest plants and new ecological niches opened up, permitting larger body-size for herbivores, omnivores and predators.[citation needed]
Amniote traits
[edit]While the early amniotes resembled their amphibian ancestors in many respects, a key difference was the lack of an otic notch at the back margin of the skull roof. In their ancestors, this notch held a spiracle, an unnecessary structure in an animal without an aquatic larval stage.[26] There are three main lines of amniotes, which may be distinguished by the structure of the skull and in particular the number of temporal fenestrae (openings) behind each eye. In anapsids, the ancestral condition, there are none, in synapsids (mammals and their extinct relatives) there is one, and most diapsids (including birds, crocodilians, squamates, and tuataras), have two. Turtles were traditionally classified as anapsids because they lack fenestrae, but molecular testing firmly places them in the diapsid line of descent – they therefore secondarily lost their fenestrae.
Post-cranial remains of amniotes can be identified from their Labyrinthodont ancestors by their having at least two pairs of sacral ribs, a sternum in the pectoral girdle (some amniotes have lost it) and an astragalus bone in the ankle.[27]
Definition and classification
[edit]Amniota was first formally described by the embryologist Ernst Haeckel in 1866 on the presence of the amnion, hence the name. A problem with this definition is that the trait (apomorphy) in question does not fossilize, and the status of fossil forms has to be inferred from other traits.
Traditional classification
[edit]Older classifications of the amniotes traditionally recognised three classes based on major traits and physiology:[29][30][31][32]
- Class Reptilia (reptiles)
- Subclass Anapsida ("proto-reptiles", possibly including turtles)
- Subclass Diapsida (majority of reptiles,[33] progenitors of birds)
- Subclass Euryapsida (plesiosaurs, placodonts, and ichthyosaurs)
- Subclass Synapsida (stem or proto-mammals, progenitors of mammals)
- Class Aves (birds)
- Subclass Archaeornithes (reptile-like birds, progenitors of all other birds)
- Subclass Enantiornithes (early birds with an alternative shoulder joint)[34]
- Subclass Hesperornithes (toothed aquatic flightless birds)
- Subclass Ichthyornithes (toothed, but otherwise modern birds)
- Subclass Neornithes (all living birds)
- Class Mammalia (mammals)
- Subclass Prototheria (Monotremata, egg-laying mammals)
- Subclass Theria (metatheria (such as marsupials) and eutheria (such as placental mammals))
This rather orderly scheme is the one most commonly found in popular and basic scientific works. It has come under critique from cladistics, as the class Reptilia is paraphyletic—it has given rise to two other classes not included in Reptilia.
Most species described as microsaurs, formerly grouped in the extinct and prehistoric amphibian group lepospondyls, has been placed in the newer clade Recumbirostra, and shares many anatomical features with amniotes which indicates they were amniotes themselves.[35]
Classification into monophyletic taxa
[edit]A different approach is adopted by writers who reject paraphyletic groupings. One such classification, by Michael Benton, is presented in simplified form below.[36]
- Series Amniota
- (Class) Clade Synapsida
- A series of unassigned families, corresponding to Pelycosauria †
- (Order) Clade Therapsida
- Class Mammalia – mammals
- (Class) Clade Sauropsida
- Subclass Parareptilia †
- Family Mesosauridae †
- Family Millerettidae †
- Family Bolosauridae †
- Family Procolophonidae †
- Order Pareiasauromorpha
- Family Nycteroleteridae †
- Family Pareiasauridae †
- (Subclass) Clade Eureptilia
- Family Captorhinidae †
- (Infraclass) Clade Diapsida
- Family Araeoscelididae †
- Family Weigeltisauridae †
- Order Younginiformes †
- (Infraclass) Clade Neodiapsida
- Order Testudinata
- Suborder Testudines – turtles
- Infraclass Lepidosauromorpha
- Unnamed infrasubclass
- Infraclass Ichthyosauria †
- Order Thalattosauria †
- Superorder Lepidosauriformes
- Order Sphenodontida – tuatara
- Order Squamata – lizards and snakes
- Infrasubclass Sauropterygia †
- Order Placodontia †
- Order Eosauropterygia †
- Suborder Pachypleurosauria †
- Suborder Nothosauria †
- Order Plesiosauria †
- Unnamed infrasubclass
- (Infraclass) Clade Archosauromorpha
- Family Trilophosauridae †
- Order Rhynchosauria †
- Order Protorosauria †
- Division Archosauriformes
- Subdivision Archosauria
- Infradivision Crurotarsi
- Order Phytosauria†
- Family Ornithosuchidae †
- Family Stagonolepididae †
- Family Rauisuchidae †
- Superfamily Poposauroidea †
- Superorder Crocodylomorpha
- Order Crocodylia – crocodilians
- Infradivision Avemetatarsalia
- Infrasubdivision Ornithodira
- Order Pterosauria †
- Family Lagerpetidae †
- Family Silesauridae †
- (Superorder) Clade Dinosauria – dinosaurs
- Order Ornithischia †
- (Order) Clade Saurischia
- Infrasubdivision Ornithodira
- Infradivision Crurotarsi
- Subdivision Archosauria
- Order Testudinata
- Subclass Parareptilia †
- (Class) Clade Synapsida
Phylogenetic classification
[edit]With the advent of cladistics, other researchers have attempted to establish new classes, based on phylogeny, but disregarding the physiological and anatomical unity of the groups. Unlike Benton, for example, Jacques Gauthier and colleagues forwarded a definition of Amniota in 1988 as "the most recent common ancestor of extant mammals and reptiles, and all its descendants".[27] As Gauthier makes use of a crown group definition, Amniota has a slightly different content than the biological amniotes as defined by an apomorphy.[37] Though traditionally considered reptiliomorphs, some recent research has recovered diadectomorphs as the sister group to Synapsida within Amniota, based on inner ear anatomy.[38][39][40]
Cladogram
[edit]The cladogram presented here illustrates the phylogeny (family tree) of amniotes, and follows a simplified version of the relationships found by Laurin & Reisz (1995),[41] with the exception of turtles, which more recent morphological and molecular phylogenetic studies placed firmly within diapsids.[42][43][44][45][46][47] The cladogram covers the group as defined under Gauthier's definition.
| Reptiliomorpha |
| ||||||||||||||||||||||||||||||||||||||||||||||||
Following studies in 2022 and 2023,[48][49] with Drepanosauromorpha placed sister to Weigeltisauridae (Coelurosauravus) in Avicephala based on Senter (2004):[50]
| |||||||||||||||||||
References
[edit]- ^ a b Marjanović, D. (2021). "The Making of Calibration Sausage Exemplified by Recalibrating the Transcriptomic Timetree of Jawed Vertebrates". Frontiers in Genetics. 12. 521693. doi:10.3389/fgene.2021.521693. PMC 8149952. PMID 34054911.
- ^ Paton, R. L.; Smithson, T. R.; Clack, J. A. (8 April 1999). "An amniote-like skeleton from the Early Carboniferous of Scotland". Nature. 398 (6727): 508–513. Bibcode:1999Natur.398..508P. doi:10.1038/19071. ISSN 0028-0836. S2CID 204992355.
- ^ Irmis, R. B.; Parker, W. G. (2005). "Unusual tetrapod teeth from the Upper Triassic Chinle Formation, Arizona, USA". Canadian Journal of Earth Sciences. 42 (7): 1339–1345. Bibcode:2005CaJES..42.1339I. doi:10.1139/e05-031. S2CID 46418796.
- ^ Laurin, Michel; Reisz, Robert R. "Amniota". RegNum. Retrieved 19 July 2024.
- ^ Queiroz, Kevin de; Cantino, Philip D.; Gauthier, Jacques A. (30 April 2020). Phylonyms: A Companion to the PhyloCode (1 ed.). CRC Press. doi:10.1201/9780429446276. ISBN 978-0-429-44627-6.
- ^ Delsuc, Frédéric; Philippe, Hervé; Tsagkogeorga, Georgia; Simion, Paul; Tilak, Marie-Ka; Turon, Xavier; López-Legentil, Susanna; Piette, Jacques; Lemaire, Patrick; Douzery, Emmanuel J. P. (13 April 2018). "A phylogenomic framework and timescale for comparative studies of tunicates". BMC Biology. 16 (1): 39. doi:10.1186/s12915-018-0499-2. ISSN 1741-7007. PMC 5899321. PMID 29653534.
- ^ Wang, Zhuo; Pascual-Anaya, Juan; Zadissa, Amonida; Li, Wenqi; Niimura, Yoshihito; Huang, Zhiyong; Li, Chunyi; White, Simon; Xiong, Zhiqiang; Fang, Dongming; Wang, Bo; Ming, Yao; Chen, Yan; Zheng, Yuan; Kuraku, Shigehiro; Pignatelli, Miguel; Herrero, Javier; Beal, Kathryn; Nozawa, Masafumi; Li, Qiye; Wang, Juan; Zhang, Hongyan; Yu, Lili; Shigenobu, Shuji; Wang, Junyi; Liu, Jiannan; Flicek, Paul; Searle, Steve; Wang, Jun; Kuratani, Shigeru; Yin, Ye; Aken, Bronwen; Zhang, Guojie; Irie, Naoki (June 2013). "The draft genomes of soft-shell turtle and green sea turtle yield insights into the development and evolution of the turtle-specific body plan". Nature Genetics. 45 (6): 701–706. doi:10.1038/ng.2615. ISSN 1546-1718. PMC 4000948. PMID 23624526.
- ^ Rygel, Michael C.; Lally, Corinne; Gibling, Martin R.; Ielpi, Alessandro; Calder, John H.; Bashforth, Arden R. (29 January 2015). "Sedimentology and stratigraphy of the type section of the Pennsylvanian Boss Point Formation, Joggins Fossil Cliffs, Nova Scotia, Canada". Atlantic Geoscience. 51: 001–043. doi:10.4138/atlgeol.2015.001. ISSN 2564-2987.
- ^ Didier, Gilles; Laurin, Michel (1 November 2020). "Exact Distribution of Divergence Times from Fossil Ages and Tree Topologies". Systematic Biology. 69 (6): 1068–1087. doi:10.1093/sysbio/syaa021. PMID 32191326.
- ^ Benton, Michael J. (1997). Vertebrate Palaeontology. London: Chapman & Hall. pp. 105–109. ISBN 978-0-412-73810-4.
- ^ Cieri, R.L., Hatch, S.T., Capano, J.G. et al. (2020). Locomotor rib kinematics in two species of lizards and a new hypothesis for the evolution of aspiration breathing in amniotes. Sci Rep 10. 7739. https://doi.org/10.1038/s41598-020-64140-y
- ^ Janis, C. M., Napoli, J. G., & Warren, D. E. (2020). Palaeophysiology of pH regulation in tetrapods. Philosophical Transactions of the Royal Society B: Biological Sciences, 375 (1793), 20190131. https://doi.org/10.1098/rstb.2019.0131
- ^ Hickman, Cleveland P. Jr (17 October 2016). Integrated principles of zoology (Seventeenth ed.). McGraw-Hill. pp. 563–567. ISBN 978-1-259-56231-0.
- ^ a b c Kardong, Kenneth V. (16 February 2011). Vertebrates: Comparative Anatomy, Function, Evolution. McGraw-Hill. ISBN 978-0-07-352423-8.
- ^ Clack, Jennifer A. (27 August 2023). Gaining Ground: The Origin and Evolution of Tetrapods. Indiana University Press. p. 370. ISBN 978-0-253-35675-8.
- ^ Mann, Arjan; Gee, Bryan M.; Pardo, Jason D.; Marjanović, David; Adams, Gabrielle R.; Calthorpe, Ami S.; Maddin, Hillary C.; Anderson, Jason S. (5 May 2020). Sansom, Robert (ed.). "Reassessment of historic 'microsaurs' from Joggins, Nova Scotia, reveals hidden diversity in the earliest amniote ecosystem". Papers in Palaeontology. 6 (4). Wiley: 605–625. Bibcode:2020PPal....6..605M. doi:10.1002/spp2.1316. ISSN 2056-2802.
- ^ a b Benton, M.J.; Donoghue, P.C.J. (2006). "Palaeontological evidence to date the tree of life". Molecular Biology and Evolution. 24 (1): 26–53. doi:10.1093/molbev/msl150. PMID 17047029.
- ^ Oxford English Dictionary
- ^ Alexander M. Schreiber; Donald D. Brown (2003). "Tadpole skin dies autonomously in response to thyroid hormone at metamorphosis". Proceedings of the National Academy of Sciences. 100 (4): 1769–1774. Bibcode:2003PNAS..100.1769S. doi:10.1073/pnas.252774999. PMC 149908. PMID 12560472.
- ^ "the_mid_palaeozoic_biotic_crisis – Ocean and Earth Science, National Oceanography Centre Southampton – University of Southampton".
- ^ Stewart J. R. (1997): Morphology and evolution of the egg of oviparous amniotes. In: S. Sumida and K. Martin (ed.) Amniote Origins-Completing the Transition to Land (1): 291–326. London: Academic Press.
- ^ Cunningham, B.; Huene, E. (July–August 1938). "Further Studies on Water Absorption by Reptile Eggs". The American Naturalist. 72 (741): 380–385. doi:10.1086/280791. JSTOR 2457547. S2CID 84258651.
- ^ Shell Game » American Scientist
- ^ Michel Laurin (2004). "The evolution of body size, Cope's rule and the origin of amniotes". Systematic Biology. 53 (4): 594–622. doi:10.1080/10635150490445706. PMID 15371249.
- ^ Gomez-Mestre, Ivan; Pyron, Robert Alexander; Wiens, John J. (2012). "Phylogenetic Analyses Reveal Unexpected Patterns in the Evolution of Reproductive Modes in Frogs". Evolution. 66 (12): 3687–3700. doi:10.1111/j.1558-5646.2012.01715.x. hdl:10261/63940. PMID 23206128.
- ^ Lombard, R. E. & Bolt, J. R. (1979): Evolution of the tetrapod ear: an analysis and reinterpretation. Biological Journal of the Linnean Society No 11: pp 19–76 Abstract
- ^ a b Gauthier, J., Kluge, A.G. and Rowe, T. (1988). "The early evolution of the Amniota." Pp. 103–155 in Benton, M.J. (ed.), The phylogeny and classification of the tetrapods, Volume 1: amphibians, reptiles, birds. Oxford: Clarendon Press.
- ^ Falcon-Lang, H J; Benton, M J; Stimson, M (2007). "Ecology of early reptiles inferred from Lower Pennsylvanian trackways". Journal of the Geological Society. 164 (6): 1113–1118. doi:10.1016/j.palaeo.2010.06.020.
- ^ Romer A S and Parsons T S (1985) The Vertebrate Body. (6th ed.) Saunders, Philadelphia.
- ^ Carroll, R. L. (1988), Vertebrate Paleontology and Evolution, WH Freeman & Co.
- ^ Hildebrand, M.; G. E. Goslow Jr (2001). Analysis of vertebrate structure. New York: Wiley. p. 429. ISBN 978-0-471-29505-1.
- ^ Colbert, E.H. & Morales, M. (2001): Colbert's Evolution of the Vertebrates: A History of the Backboned Animals Through Time. 4th edition. John Wiley & Sons, Inc, New York — ISBN 978-0-471-38461-8.
- ^ Reeder, Tod W.; Townsend, Ted M.; Mulcahy, Daniel G.; Noonan, Brice P.; Wood, Perry L.; Sites, Jack W.; Wiens, John J. (2015). "Integrated Analyses Resolve Conflicts over Squamate Reptile Phylogeny and Reveal Unexpected Placements for Fossil Taxa". PLOS ONE. 10 (3): e0118199. Bibcode:2015PLoSO..1018199R. doi:10.1371/journal.pone.0118199. PMC 4372529. PMID 25803280.
- ^ *Hope, S. (2002) The Mesozoic record of Neornithes (modern birds). In: Chiappe, L.M. and Witmer, L.M. (eds.): Mesozoic Birds: Above the Heads of Dinosaurs: 339–388. University of California Press, Berkeley. ISBN 0-520-20094-2
- ^ Tiny ancient reptile named after Thor's world-ending nemesis
- ^ Benton, M.J. (2015). "Appendix: Classification of the Vertebrates". Vertebrate Paleontology (4th ed.). Wiley Blackwell. 433–447. ISBN 978-1-118-40684-7.
- ^ Lee, M.S.Y. & Spencer, P.S. (1997): Crown clades, key characters and taxonomic stability: when is an amniote not an amniote? In: Sumida S.S. & Martin K.L.M. (eds.) Amniote Origins: completing the transition to land. Academic Press, pp 61–84. Google books
- ^ David S. Berman (2013). "Diadectomorphs, amniotes or not?". New Mexico Museum of Natural History and Science Bulletin. 60: 22–35.
- ^ Jozef Klembara; Miroslav Hain; Marcello Ruta; David S. Berman; Stephanie E. Pierce; Amy C. Henrici (2019). "Inner ear morphology of diadectomorphs and seymouriamorphs (Tetrapoda) uncovered by high-resolution x-ray microcomputed tomography, and the origin of the amniote crown group" (PDF). Palaeontology. 63: 131–154. doi:10.1111/pala.12448.
- ^ Klembara, Jozef; Ruta, Marcello; Hain, Miroslav; Berman, David S. (2021). "Braincase and Inner Ear Anatomy of the Late Carboniferous Tetrapod Limnoscelis dynatis (Diadectomorpha) Revealed by High-Resolution X-ray Microcomputed Tomography" (PDF). Frontiers in Ecology and Evolution. 9. doi:10.3389/fevo.2021.709766. ISSN 2296-701X.
- ^ Laurin, M.; Reisz, R.R. (1995). "A reevaluation of early amniote phylogeny" (PDF). Zoological Journal of the Linnean Society. 113 (2): 165–223. doi:10.1111/j.1096-3642.1995.tb00932.x. Archived from the original (PDF) on 8 June 2019. Retrieved 2 November 2017.
- ^ Rieppel, O.; DeBraga, M. (1996). "Turtles as diapsid reptiles" (PDF). Nature. 384 (6608): 453–5. Bibcode:1996Natur.384..453R. doi:10.1038/384453a0. S2CID 4264378.
- ^ Müller, Johannes (2004). "The relationships among diapsid reptiles and the influence of taxon selection". In Arratia, G; Wilson, M.V.H.; Cloutier, R. (eds.). Recent Advances in the Origin and Early Radiation of Vertebrates. Verlag Dr. Friedrich Pfeil. pp. 379–408. ISBN 978-3-89937-052-2.
- ^ Tyler R. Lyson; Erik A. Sperling; Alysha M. Heimberg; Jacques A. Gauthier; Benjamin L. King; Kevin J. Peterson (23 February 2012). "MicroRNAs support a turtle + lizard clade". Biology Letters. 8 (1): 104–107. doi:10.1098/rsbl.2011.0477. PMC 3259949. PMID 21775315.
- ^ Iwabe, N.; Hara, Y.; Kumazawa, Y.; Shibamoto, K.; Saito, Y.; Miyata, T.; Katoh, K. (29 December 2004). "Sister group relationship of turtles to the bird-crocodilian clade revealed by nuclear DNA-coded proteins". Molecular Biology and Evolution. 22 (4): 810–813. doi:10.1093/molbev/msi075. PMID 15625185.
- ^ Roos, Jonas; Aggarwal, Ramesh K.; Janke, Axel (November 2007). "Extended mitogenomic phylogenetic analyses yield new insight into crocodylian evolution and their survival of the Cretaceous–Tertiary boundary". Molecular Phylogenetics and Evolution. 45 (2): 663–673. Bibcode:2007MolPE..45..663R. doi:10.1016/j.ympev.2007.06.018. PMID 17719245.
- ^ Katsu, Y.; Braun, E. L.; Guillette, L. J. Jr.; Iguchi, T. (17 March 2010). "From reptilian phylogenomics to reptilian genomes: analyses of c-Jun and DJ-1 proto-oncogenes". Cytogenetic and Genome Research. 127 (2–4): 79–93. doi:10.1159/000297715. PMID 20234127. S2CID 12116018.
- ^ Simões, T. R.; Kammerer, C. F.; Caldwell, M. W.; Pierce, S. E. (2022). "Successive climate crises in the deep past drove the early evolution and radiation of reptiles". Science Advances. 8 (33): eabq1898. Bibcode:2022SciA....8.1898S. doi:10.1126/sciadv.abq1898. PMC 9390993. PMID 35984885.
- ^ Wolniewicz, Andrzej S; Shen, Yuefeng; Li, Qiang; Sun, Yuanyuan; Qiao, Yu; Chen, Yajie; Hu, Yi-Wei; Liu, Jun (8 August 2023). Ibrahim, Nizar (ed.). "An armoured marine reptile from the Early Triassic of South China and its phylogenetic and evolutionary implications". eLife. 12: e83163. doi:10.7554/eLife.83163. ISSN 2050-084X. PMC 10499374. PMID 37551884.
- ^ Senter, Phil (January 2004). "Phylogeny of Drepanosauridae (Reptilia: Diapsida)". Journal of Systematic Palaeontology. 2 (3): 257–268. Bibcode:2004JSPal...2..257S. doi:10.1017/S1477201904001427. ISSN 1477-2019. S2CID 83840423.