Germanium(II) iodide
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.620 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| GeI2 | |
| Molar mass | 326.439 g·mol−1 |
| Appearance | yellow solid[1] |
| Density | 5.37 g·cm−3 (25 °C)[2] |
| Melting point | 428 °C[3] |
| Boiling point | 550 °C (decomposes)[3] |
| Structure | |
| P3m1 (No. 164)[4] | |
| Related compounds | |
Other anions
|
germanium(II) fluoride germanium(II) chloride germanium(II) bromide |
Other cations
|
tin(II) iodide lead(II) iodide |
Related compounds
|
germanium(IV) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Germanium(II) iodide is an iodide of germanium, with the chemical formula of GeI2.
Preparation
[edit]Germanium(II) iodide can be produced by reacting germanium(IV) iodide with hydriodic acid and hypophosphorous acid and water:[1]
- GeI4 + H2O + H3PO2 → GeI2 + H3PO3 + 2 HI
It can also be formed by the reaction of germanium monosulfide or germanium monoxide and hydrogen iodide.[1]
- GeO + 2 HI → GeI2 + H2O
- GeS + 2 HI → GeI2 + H2S}
It can also be produced from the direct reaction of germanium and iodine at 200 – 400 °C:[1]
- Ge + I2 → GeI2
Germanium(II) iodide can also be formed from the decomposition of HGeI3, which can be prepared by reacting HGeCl3 with hydroiodic acid:[5]
- HGeCl3 + 3 HI → HGeI3 + HCl
- HGeI3 → GeI2 + HI
Properties
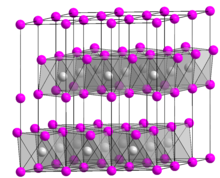
[edit]Germanium(II) iodide is a yellow crystal that slowly hydrolyzes into germanium(II) hydroxide in the presence of moisture. It is insoluble in hydrocarbons and slightly soluble in chloroform and carbon tetrachloride. It has a cadmium iodide structure with lattice parameters a = 413 pm and c = 679 pm.[1] It disproportionates to germanium and germanium tetraiodide at 550 °C.[6]
Applications
[edit]Germanium(II) iodide can react with carbene to form stable compounds.[2] It is also used in the electronics industry to produce germanium layers epitaxially through disproportionation reactions.[7]
References
[edit]- ^ a b c d e Georg Brauer (Hrsg.), unter Mitarbeit von Marianne Baudler u. a.: Handbuch der Präparativen Anorganischen Chemie. 3., umgearbeitete Auflage. Band I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6, S. 727.
- ^ a b Sigma-Aldrich Co., product no. {{{id}}}.
- ^ a b William M. Haynes (2012), CRC Handbook of Chemistry and Physics, 93rd Edition, CRC Press, pp. 4–65, ISBN 978-143988049-4
- ^ Jean d’Ans, Ellen Lax, Roger Blachnik (1998), Taschenbuch für Chemiker und Physiker, Springer DE, p. 472, ISBN 364258842-5
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Wolfgang Kirmse (2013), Carbene Chemistry 2e, Elsevier, p. 540, ISBN 978-032316145-9
- ^ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, p. 959, ISBN 0-12-352651-5
- ^ A.G. Milnes (1972), Heterojunctions and Metal Semiconductor Junctions, Elsevier, p. 104, ISBN 032314136-6
