Iron(III) citrate
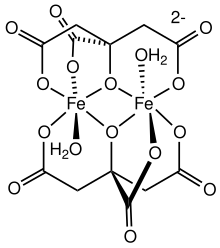 One of several ferric citrate complexes[1]
| |
| Names | |
|---|---|
| IUPAC name
iron(3+) 2-hydroxypropane-1,2,3-tricarboxylate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.020.488 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H5FeO7 | |
| Molar mass | 244.944 g·mol−1 |
| Appearance | dark orange-red brown solid[2] |
| ~5 g/L in water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ferric citrate or iron(III) citrate describes any of several complexes formed upon binding any of the several conjugate bases derived from citric acid with ferric ions. Most of these complexes are orange or red-brown. They contain two or more Fe(III) centers.[3]
Ferric citrates contribute to the metabolism of iron by some organisms. Citrates, which are released by plant roots and by some microorganisms, can solubilize iron compounds in the soil. For example ferric hydroxide reacts with citrates to give form soluble complexes. This solubilization provides a pathway for the absorption of the ferric ions by various organisms.[4]
Ferric citrate is used in medicine to regulate the blood levels of iron in patients with chronic kidney disease on dialysis. It acts by forming an insoluble compound with phosphate present in the diet and thus minimizing its uptake by the digestive system.[5]
Structure
[edit]Citrate forms a variety of coordination complexes with ferric ions.[6][1] Some might be oligomers, and polymers. Thus, ferric citrate is not a single well-defined compound, but a family of compounds, many with similar formulas. These various forms can coexist in equilibrium.[7] At physiological pH, ferric citrate forms an insoluble red polymer. In other conditions, it forms anionic complexes like [FeC
6H
4O
7]2(H
2O)2]2−. In the present of excess citrate anions, the iron forms negatively charged complexes like [Fe(C
6H
4O
7)2]5− and [Fe
9O(C
6H
4O
7)8(H
2O)3]7−.[3][4]
Photoreduction
[edit]The Fe3+
ion in ferric citrate (as in many iron(III) carboxylates) is reduced by exposure to light,[8] especially blue and ultraviolet, to Fe2+
(ferrous) ion with concomitant oxidation of the carboxyl group adjacent to the hydroxyl, yielding carbon dioxide and acetonedicarboxylate:
- 2Fe3+
+ R2-C(OH)-CO−
2 → 2Fe2+
+ R2-C=O + H+
+ CO
2
where -R represents the group -CH
2CO−
2.
This reaction plays an important role in plant metabolism: iron is carried up from the roots as ferric citrate dissolved in the sap,[9]
and photoreduced in the leaves to iron(II) that can be transported into the cells.
Additional reading
[edit]Abrahamson, Harmon B.; Rezvani, Ahmad B.; Brushmiller, J.George (1994). "Photochemical and Spectroscopic Studies of Complexes, of Iron(III) with Citric Acid and Other Carboxylic Acids". Inorganica Chimica Acta. 226 (1–2): 117–127. doi:10.1016/0020-1693(94)04077-X.
See also
[edit]References
[edit]- ^ a b Shweky, Itzhak; Bino, Avi; Goldberg, David P.; Lippard, Stephen J. (1994). "Syntheses, Structures, and Magnetic Properties of Two Dinuclear Iron(III) Citrate Complexes". Inorganic Chemistry. 33 (23): 5161–5162. doi:10.1021/ic00101a001.
- ^ Sigma-Aldrich: Product Specification - Iron(III) citrate, technical grade. Accessed on 2017-03-09.
- ^ a b Bino, Avi; Shweky, Itzhak; Cohen, Shmuel; Bauminger, Erika R.; Lippard, Stephen J. (1998). "A Novel Nonairon(III) Citrate Complex: A "Ferric Triple-Decker"". Inorganic Chemistry. 37 (20): 5168–5172. doi:10.1021/ic9715658.
- ^ a b Pierre, J. L.; Gautier-Luneau, I. (2000). "Iron and Citric Acid: A Fuzzy Chemistry of Ubiquitous Biological Relevance". Biometals. 13 (1): 91–96. doi:10.1023/A:1009225701332. PMID 10831230. S2CID 2301450.
- ^ Lewis, Julia B.; Sika, Mohammed; Koury, Mark J.; Chuang, Peale; Schulman, Gerald; Smith, Mark T.; Whittier, Frederick C.; Linfert, Douglas R.; Galphin, Claude M.; Athreya, Balaji P.; Nossuli, A. Kaldun Kaldun; Chang, Ingrid J.; Blumenthal, Samuel S.; Manley, John; Zeig, Steven; Kant, Kotagal S.; Olivero, Juan Jose; Greene, Tom; Dwyer, Jamie P.; Collaborative Study Group (2015). "Ferric Citrate Controls Phosphorus and Delivers Iron in Patients on Dialysis". Journal of the American Society of Nephrology. 26 (2): 493–503. doi:10.1681/ASN.2014020212. PMC 4310662. PMID 25060056.
- ^ Xiang Hao, Yongge Wei, Shiwei Zhang (2001): "Synthesis, crystal structure and magnetic property of a binuclear iron(III) citrate complex". Transition Metal Chemistry, volume 26, issue 4, pages 384–387. doi:10.1023/A:1011055306645
- ^ Silva, Andre M. N.; Kong, Xiaole; Parkin, Mark C.; Cammack, Richard; Hider, Robert C. (2009). "Iron(III) citrate speciation in aqueous solution". Dalton Transactions (40): 8616–25. doi:10.1039/B910970F. PMID 19809738.
- ^ Wu Feng and Deng Nansheng (2000): "Photochemistry of hydrolytic iron (III) species and photoinduced degradation of organic compounds: A minireview". Chemosphere, volume 41, issue 8, pages 1137–1147. doi:10.1016/S0045-6535(00)00024-2
- ^ Rellán-Álvarez, Rubén; Giner-Martínez-Sierra, Justo; Orduna, Jesús; Orera, Irene; Rodríguez-Castrillón, José Ángel; García-Alonso, José Ignacio; Abadía, Javier; Álvarez-Fernández, Ana (2010). "Identification of a Tri-Iron(III), Tri-Citrate Complex in the Xylem Sap of Iron-Deficient Tomato Resupplied with Iron: New Insights into Plant Iron Long-Distance Transport". Plant and Cell Physiology. 51 (1): 91–102. doi:10.1093/pcp/pcp170. PMID 19942594.
