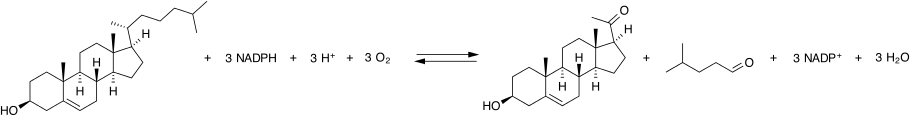
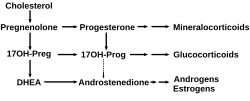
Cholesterol side-chain cleavage enzyme
Cholesterol side-chain cleavage enzyme is commonly referred to as P450scc, where "scc" is an abbreviation for side-chain cleavage. P450scc is a mitochondrial enzyme that catalyzes conversion of cholesterol to pregnenolone. This is the first reaction in the process of steroidogenesis in all mammalian tissues that specialize in the production of various steroid hormones.[5]
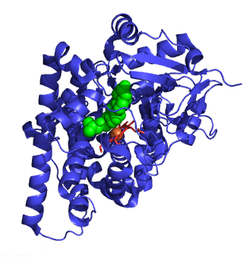
P450scc is a member of the cytochrome P450 superfamily of enzymes (family 11, subfamily A, polypeptide 1) and is encoded by the CYP11A1 gene.[6]
Nomenclature
[edit]| cholesterol monooxygenase (side-chain-cleaving) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 1.14.15.6 | ||||||||
| CAS no. | 37292-81-2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
The systematic name of this enzyme class is cholesterol, reduced-adrenal-ferredoxin:oxygen oxidoreductase (side-chain-cleaving). Other names include:
|
Tissue and intracellular localization
[edit]The highest level of the cholesterol side-chain cleavage system is found in the adrenal cortex and the corpus luteum.[5] The system is also expressed at high levels in steroidogenic theca cells in the ovary, and Leydig cells in the testis.[5] During pregnancy, the placenta also expresses significant levels of this enzyme system.[7] P450scc is also present at much lower levels in several other tissue types, including the brain.[8] In the adrenal cortex, the concentration of adrenodoxin is similar to that of P450scc, but adrenodoxin reductase is expressed at lower levels.[9]
Immunofluorescence studies using specific antibodies against P450scc system enzymes have demonstrated that proteins are located exclusively within the mitochondria.[10][11] P450scc is associated with the inner mitochondrial membrane, facing the interior (matrix).[12][13] Adrenodoxin and adrenodoxin reductase are soluble peripheral membrane proteins located inside the mitochondrial matrix that appear to associate with each other primarily through electrostatic interactions.[14]
Mechanism of action
[edit]P450scc catalyzes the conversion of cholesterol to pregnenolone in three monooxygenase reactions. These involve 2 hydroxylations of the cholesterol side-chain, which generate, first, 22R-hydroxycholesterol and then 20alpha,22R-dihydroxycholesterol. The final step cleaves the bond between carbons 20 and 22, resulting in the production of pregnenolone and isocaproic aldehyde.
Each monooxygenase step requires 2 electrons (reducing equivalents). The initial source of the electrons is NADPH.[15] The electrons are transferred from NADPH to P450scc via two electron transfer proteins: adrenodoxin reductase[16] and adrenodoxin.[17][18] All three proteins together constitute the cholesterol side-chain cleavage complex.
The involvement of three proteins in cholesterol side-chain cleavage reaction raises the question of whether the three proteins function as a ternary complex as reductase:adrenodoxin:P450. Both spectroscopic studies of adrenodoxin binding to P450scc and kinetic studies in the presence of varying concentrations of adrenodoxin reductase demonstrated that the reductase competes with P450scc for binding to adrenodoxin. These results demonstrated that the formation of a functional ternary complex is not possible.[17] From these studies, it was concluded that the binding sites of adrenodoxin to its reductase and to P450 are overlapping and, as a consequence, adrenodoxin functions as a mobile electron shuttle between reductase and P450.[17] These conclusions have been confirmed by structural analysis of adrenodoxin and P450 complex.[19]
The process of electron transfer from NADPH to P450scc is not tightly coupled; that is, during electron transfer from adrenodoxin reductase via adrenodoxin to P450scc, a certain portion of the electrons leak outside of the chain and react with O2, generating superoxide radicals.[20] Steroidogenic cells include a diverse array of antioxidant systems to cope with the radicals generated by the steroidogenic enzymes.[21]
Regulation
[edit]In each steroidogenic cell, the expression of the P450scc system proteins is regulated by the trophic hormonal system specific for the cell type.[5] In adrenal cortex cells from zona fasciculata, the expression of the mRNAs encoding all three P450scc proteins is induced by corticotropin (ACTH).[11][22] The trophic hormones increase CYP11A1 gene expression through transcription factors such as steroidogenic factor 1 (SF-1), by the α isoform of activating protein 2 (AP-2) in the human, and many others.[22][23] The production of this enzyme is inhibited notably by the nuclear receptor DAX-1.[22]
P450scc is always active, however its activity is limited by the supply of cholesterol in the inner membrane. The supplying of cholesterol to this membrane (from the outer mitochondrial membrane) is, thus, considered the true rate-limiting step in steroid production. This step is mediated primarily by the steroidogenic acute regulatory protein (StAR or STARD1). Upon stimulation of a cell to make steroid, the amount of StAR available to transfer cholesterol to the inner membrane limits how fast the reaction can go (the acute phase). With prolonged (chronic) stimulation, it is thought that cholesterol supply becomes no longer an issue and that the capacity of the system to make steroid (i.e., level of P450scc in the mitochondria) is now more important.
Corticotropin (ACTH) is a hormone that is released from the anterior pituitary in response to stress situations. A study of the steroidogenic capacity of the adrenal cortex in infants with acute respiratory disease demonstrated that indeed during disease state there is a specific increase in the steroidogenic capacity for the synthesis of the glucocorticoid cortisol but not for the mineralocorticoid aldosterone or androgen DHEAS that are secreted from other zones of the adrenal cortex.[24]
Pathology
[edit]Mutations in the CYP11A1 gene result in a steroid hormone deficiency, causing a minority of cases of the rare and potentially fatal condition lipoid congenital adrenal hyperplasia.[25][26][27] Deficiency of CYP11A1 can result in hyperpigmentation, hypoglycemia, and recurrent infections.[28]
Inhibitors
[edit]Cholesterol side-chain cleavage enzyme inhibitors include aminoglutethimide, ketoconazole, and mitotane, among others.[29][30][31]
See also
[edit]References
[edit]- ^ a b c ENSG00000288362 GRCh38: Ensembl release 89: ENSG00000140459, ENSG00000288362 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000032323 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b c d Hanukoglu I (December 1992). "Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis". The Journal of Steroid Biochemistry and Molecular Biology. 43 (8): 779–804. doi:10.1016/0960-0760(92)90307-5. PMID 22217824. S2CID 112729.
- ^ "Entrez Gene: CYP11A1 cytochrome P450, family 11, subfamily A, polypeptide 1".
- ^ Strauss JF, Martinez F, Kiriakidou M (February 1996). "Placental steroid hormone synthesis: unique features and unanswered questions". Biology of Reproduction. 54 (2): 303–311. doi:10.1095/biolreprod54.2.303. PMID 8788180.
- ^ Stoffel-Wagner B (December 2001). "Neurosteroid metabolism in the human brain". European Journal of Endocrinology. 145 (6): 669–679. doi:10.1530/eje.0.1450669. PMID 11720889.
- ^ Hanukoglu I, Hanukoglu Z (May 1986). "Stoichiometry of mitochondrial cytochromes P-450, adrenodoxin and adrenodoxin reductase in adrenal cortex and corpus luteum. Implications for membrane organization and gene regulation". European Journal of Biochemistry. 157 (1): 27–31. doi:10.1111/j.1432-1033.1986.tb09633.x. PMID 3011431.
- ^ Hanukoglu I, Suh BS, Himmelhoch S, Amsterdam A (October 1990). "Induction and mitochondrial localization of cytochrome P450scc system enzymes in normal and transformed ovarian granulosa cells". The Journal of Cell Biology. 111 (4): 1373–1381. doi:10.1083/jcb.111.4.1373. PMC 2116250. PMID 2170421.
- ^ a b Hanukoglu I, Feuchtwanger R, Hanukoglu A (November 1990). "Mechanism of corticotropin and cAMP induction of mitochondrial cytochrome P450 system enzymes in adrenal cortex cells". The Journal of Biological Chemistry. 265 (33): 20602–20608. doi:10.1016/S0021-9258(17)30545-8. PMID 2173715.
- ^ Topological studies of cytochromes P-450scc and P-45011 beta in bovine adrenocortical inner mitochondrial membranes. Effects of controlled tryptic digestion. J. Biol. Chem. 1979 254: 10443-8.
- ^ Farkash Y, Timberg R, Orly J (April 1986). "Preparation of antiserum to rat cytochrome P-450 cholesterol side chain cleavage, and its use for ultrastructural localization of the immunoreactive enzyme by protein A-gold technique". Endocrinology. 118 (4): 1353–1365. doi:10.1210/endo-118-4-1353. PMID 3948785.
- ^ Hanukoglu I, Privalle CT, Jefcoate CR (May 1981). "Mechanisms of ionic activation of adrenal mitochondrial cytochromes P-450scc and P-45011 beta". The Journal of Biological Chemistry. 256 (9): 4329–4335. doi:10.1016/S0021-9258(19)69437-8. PMID 6783659.
- ^ Hanukoglu I, Rapoport R (1995). "Routes and regulation of NADPH production in steroidogenic mitochondria". Endocrine Research. 21 (1–2): 231–241. doi:10.3109/07435809509030439. PMID 7588385.
- ^ Hanukoglu I, Gutfinger T, Haniu M, Shively JE (December 1987). "Isolation of a cDNA for adrenodoxin reductase (ferredoxin-NADP+ reductase). Implications for mitochondrial cytochrome P-450 systems". European Journal of Biochemistry. 169 (3): 449–455. doi:10.1111/j.1432-1033.1987.tb13632.x. PMID 3691502.
- ^ a b c Hanukoglu I, Jefcoate CR (April 1980). "Mitochondrial cytochrome P-450scc. Mechanism of electron transport by adrenodoxin". The Journal of Biological Chemistry. 255 (7): 3057–3061. doi:10.1016/S0021-9258(19)85851-9. PMID 6766943.
- ^ Hanukoglu I, Spitsberg V, Bumpus JA, Dus KM, Jefcoate CR (May 1981). "Adrenal mitochondrial cytochrome P-450scc. Cholesterol and adrenodoxin interactions at equilibrium and during turnover". The Journal of Biological Chemistry. 256 (9): 4321–4328. doi:10.1016/S0021-9258(19)69436-6. PMID 7217084.
- ^ Strushkevich N, MacKenzie F, Cherkesova T, Grabovec I, Usanov S, Park HW (June 2011). "Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system". Proceedings of the National Academy of Sciences of the United States of America. 108 (25): 10139–10143. Bibcode:2011PNAS..10810139S. doi:10.1073/pnas.1019441108. PMC 3121847. PMID 21636783.
- ^ Hanukoglu I, Rapoport R, Weiner L, Sklan D (September 1993). "Electron leakage from the mitochondrial NADPH-adrenodoxin reductase-adrenodoxin-P450scc (cholesterol side chain cleavage) system". Archives of Biochemistry and Biophysics. 305 (2): 489–498. doi:10.1006/abbi.1993.1452. PMID 8396893.
- ^ Hanukoglu I (2006). "Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells". Drug Metabolism Reviews. 38 (1–2): 171–196. doi:10.1080/03602530600570040. PMID 16684656. S2CID 10766948.
- ^ a b c Lavoie HA, King SR (August 2009). "Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B". Experimental Biology and Medicine. 234 (8): 880–907. doi:10.3181/0903-MR-97. PMID 19491374. S2CID 5350278.
- ^ Guo IC, Shih MC, Lan HC, Hsu NC, Hu MC, Chung BC (July 2007). "Transcriptional regulation of human CYP11A1 in gonads and adrenals". Journal of Biomedical Science. 14 (4): 509–515. doi:10.1007/s11373-007-9177-z. PMID 17594537.
- ^ Hanukoglu A, Fried D, Nakash I, Hanukoglu I (November 1995). "Selective increases in adrenal steroidogenic capacity during acute respiratory disease in infants". European Journal of Endocrinology. 133 (5): 552–556. doi:10.1530/eje.0.1330552. PMID 7581984. S2CID 44439040.
- ^ Bhangoo A, Anhalt H, Ten S, King SR (March 2006). "Phenotypic variations in lipoid congenital adrenal hyperplasia". Pediatric Endocrinology Reviews. 3 (3): 258–271. PMID 16639391.
- ^ al Kandari H, Katsumata N, Alexander S, Rasoul MA (August 2006). "Homozygous mutation of P450 side-chain cleavage enzyme gene (CYP11A1) in 46, XY patient with adrenal insufficiency, complete sex reversal, and agenesis of corpus callosum". The Journal of Clinical Endocrinology and Metabolism. 91 (8): 2821–2826. doi:10.1210/jc.2005-2230. PMID 16705068.
- ^ Kim CJ, Lin L, Huang N, Quigley CA, AvRuskin TW, Achermann JC, Miller WL (March 2008). "Severe combined adrenal and gonadal deficiency caused by novel mutations in the cholesterol side chain cleavage enzyme, P450scc". The Journal of Clinical Endocrinology and Metabolism. 93 (3): 696–702. doi:10.1210/jc.2007-2330. PMC 2266942. PMID 18182448.
- ^ FBuonocore F, Achermann JC (2020). "Primary adrenal insufficiency: New genetic causes and their long-term consequences". Clinical Endocrinology. 92 (1): 11–20. doi:10.1111/cen.14109. PMC 6916405. PMID 31610036.
- ^ Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 735–. ISBN 978-0-7817-1750-2.
- ^ Jameson JL, De Groot LJ (18 May 2010). Endocrinology - E-Book: Adult and Pediatric. Elsevier Health Sciences. pp. 301–302. ISBN 978-1-4557-1126-0.
- ^ Ortiz de Montellano PR (13 March 2015). Cytochrome P450: Structure, Mechanism, and Biochemistry. Springer. pp. 851–879. ISBN 978-3-319-12108-6.
Further reading
[edit]- Helmberg A (August 1993). "Twin genes and endocrine disease: CYP21 and CYP11B genes". Acta Endocrinologica. 129 (2): 97–108. doi:10.1530/acta.0.1290097. PMID 8372604.
- Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, et al. (January 1997). "Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis". Steroids. 62 (1): 21–28. doi:10.1016/S0039-128X(96)00154-7. PMID 9029710. S2CID 1977513.
- Stocco DM (June 2000). "Intramitochondrial cholesterol transfer". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1486 (1): 184–197. doi:10.1016/S1388-1981(00)00056-1. PMID 10856721.
- Kristensen VN, Kure EH, Erikstein B, Harada N, Børresen-Dale A (October 2001). "Genetic susceptibility and environmental estrogen-like compounds". Mutation Research. 482 (1–2): 77–82. doi:10.1016/S0027-5107(01)00212-3. PMID 11535251.
- Strauss JF (November 2003). "Some new thoughts on the pathophysiology and genetics of polycystic ovary syndrome". Annals of the New York Academy of Sciences. 997 (1): 42–48. Bibcode:2003NYASA.997...42S. doi:10.1196/annals.1290.005. PMID 14644808. S2CID 23559461.
- Wada A, Waterman MR (November 1992). "Identification by site-directed mutagenesis of two lysine residues in cholesterol side chain cleavage cytochrome P450 that are essential for adrenodoxin binding". The Journal of Biological Chemistry. 267 (32): 22877–22882. doi:10.1016/S0021-9258(18)50028-4. PMID 1429635.
- Hu MC, Guo IC, Lin JH, Chung BC (March 1991). "Regulated expression of cytochrome P-450scc (cholesterol-side-chain cleavage enzyme) in cultured cell lines detected by antibody against bacterially expressed human protein". The Biochemical Journal. 274 (Pt 3): 813–817. doi:10.1042/bj2740813. PMC 1149983. PMID 1849407.
- Sparkes RS, Klisak I, Miller WL (June 1991). "Regional mapping of genes encoding human steroidogenic enzymes: P450scc to 15q23-q24, adrenodoxin to 11q22; adrenodoxin reductase to 17q24-q25; and P450c17 to 10q24-q25". DNA and Cell Biology. 10 (5): 359–365. doi:10.1089/dna.1991.10.359. PMID 1863359.
- Coghlan VM, Vickery LE (October 1991). "Site-specific mutations in human ferredoxin that affect binding to ferredoxin reductase and cytochrome P450scc". The Journal of Biological Chemistry. 266 (28): 18606–18612. doi:10.1016/S0021-9258(18)55106-1. PMID 1917982.
- Matteson KJ, Chung BC, Urdea MS, Miller WL (April 1986). "Study of cholesterol side-chain cleavage (20,22 desmolase) deficiency causing congenital lipoid adrenal hyperplasia using bovine-sequence P450scc oligodeoxyribonucleotide probes". Endocrinology. 118 (4): 1296–1305. doi:10.1210/endo-118-4-1296. PMID 2419119.
- Chung BC, Matteson KJ, Voutilainen R, Mohandas TK, Miller WL (December 1986). "Human cholesterol side-chain cleavage enzyme, P450scc: cDNA cloning, assignment of the gene to chromosome 15, and expression in the placenta". Proceedings of the National Academy of Sciences of the United States of America. 83 (23): 8962–8966. Bibcode:1986PNAS...83.8962C. doi:10.1073/pnas.83.23.8962. PMC 387054. PMID 3024157.
- Morohashi K, Sogawa K, Omura T, Fujii-Kuriyama Y (April 1987). "Gene structure of human cytochrome P-450(SCC), cholesterol desmolase". Journal of Biochemistry. 101 (4): 879–887. doi:10.1093/oxfordjournals.jbchem.a121955. PMID 3038854.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–174. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Gharani N, Waterworth DM, Batty S, White D, Gilling-Smith C, Conway GS, et al. (March 1997). "Association of the steroid synthesis gene CYP11a with polycystic ovary syndrome and hyperandrogenism". Human Molecular Genetics. 6 (3): 397–402. doi:10.1093/hmg/6.3.397. PMID 9147642.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–156. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Hukkanen J, Mäntylä M, Kangas L, Wirta P, Hakkola J, Paakki P, et al. (February 1998). "Expression of cytochrome P450 genes encoding enzymes active in the metabolism of tamoxifen in human uterine endometrium". Pharmacology & Toxicology. 82 (2): 93–97. doi:10.1111/j.1600-0773.1998.tb01404.x. PMID 9498238.
- Zhou Z, Shackleton CH, Pahwa S, White PC, Speiser PW (March 1998). "Prominent sex steroid metabolism in human lymphocytes". Molecular and Cellular Endocrinology. 138 (1–2): 61–69. doi:10.1016/S0303-7207(98)00052-5. PMID 9685215. S2CID 20490901.
Steroid hormone synthesis
[edit]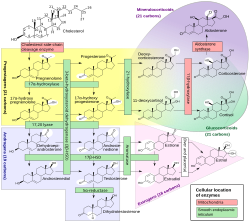
Additional images
[edit]External links
[edit]- Cytochrome+P450scc at the U.S. National Library of Medicine Medical Subject Headings (MeSH)