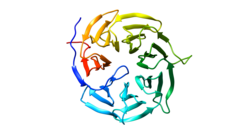CDC20
The cell division cycle protein 20 homolog is an essential regulator of cell division that is encoded by the CDC20 gene[5][6] in humans. To the best of current knowledge its most important function is to activate the anaphase promoting complex (APC/C), a large 11-13 subunit complex that initiates chromatid separation and entrance into anaphase. The APC/CCdc20 protein complex has two main downstream targets. Firstly, it targets securin for destruction, enabling the eventual destruction of cohesin and thus sister chromatid separation. It also targets S and M-phase (S/M) cyclins for destruction, which inactivates S/M cyclin-dependent kinases (Cdks) and allows the cell to exit from mitosis. A closely related protein, Cdc20homologue-1 (Cdh1) plays a complementary role in the cell cycle.
CDC20 appears to act as a regulatory protein interacting with many other proteins at multiple points in the cell cycle. It is required for two microtubule-dependent processes: nuclear movement prior to anaphase, and chromosome separation.[7]
Discovery
[edit]CDC20, along with a handful of other Cdc proteins, was discovered in the early 1970s when Hartwell and colleagues made cell-division cycle mutants that failed to complete major events in the cell cycle in the yeast strain S. cerevisiae.[8] Hartwell found mutants that did not enter anaphase and thus could not complete mitosis; this phenotype could be traced back to the CDC20 gene.[9] However, even after the biochemistry of the protein was eventually elucidated, the molecular role of CDC20 remained elusive until the discovery of the APC/C in 1995.[10][11]
Structure
[edit]CDC20 is a protein related to the beta subunit of heterotrimeric G proteins. Near its C-terminus it contains seven WD40 repeats, which are multiple short, structural motifs of around 40 amino acids that often play a role in binding with larger protein complexes. In the case of CDC20, they arrange into a seven-bladed beta propeller. The human CDC20 is about 499 amino acids long, and contains at least four phosphorylation sites near the N-terminus. In between these phosphorylation sites, which play regulatory roles, are the C-box, the KEN-box, the Mad2-interacting motif, and the Cry box. The KEN-box, as well as the Cry box, are important recognition and degradation sequences for the APC/CCdh1 complex (see below).
Interactions
[edit]CDC20 has been shown to interact with:
- ANAPC7[12][13]
- BUB1B,[13][14][15][16][17][18]
- CDC16,[12][13][16][19]
- CDC27,[12][13][16][19][20][21][22]
- Cyclin A1,[23]
- FBXO5,[24]
- HDAC1,[25]
- HDAC2,[25] and
- MAD2L1.[13][16][18][19][20][21][26][27][28][29][30][31]
However, the most important interaction of CDC20 is with the Anaphase Promoting Complex. The APC/C is a large E3 ubiquitin ligase, which triggers the metaphase to anaphase transition by marking select proteins for degradation. The two main targets of the APC/C are the S/M cyclins and the protein securin. S/M cyclins activate cyclin-dependent kinases (Cdks), which have a vast array of downstream effects that work to guide the cell through mitosis. They must be degraded for cells to exit mitosis. Securin is a protein that inhibits separase, which in turn inhibits cohesin, a protein that holds sister chromatids together. Therefore, in order for anaphase to progress, securin must be inhibited so that cohesin can be cleaved by separase. These processes are dependent on both the APC/C and CDC20: When Cdks phosphorylate the APC/C, CDC20 can bind and activate it, allowing both the degradation of Cdks and the cleavage of cohesin. APC/C activity is dependent on CDC20 (and Cdh1), because CDC20 often binds the APC/C substrates directly.[32] In fact, it is thought that CDC20 and Cdh1 (see below) are receptors for the KEN-box and D-box motifs on substrates.[33] However, these sequences are normally not sufficient for ubiquitination and degradation; much remains to be learned about how CDC20 binds its substrate.
Regulation
[edit]The APC/CCdc20 complex regulates itself so that it is present during the appropriate times of the cell cycle. In order for CDC20 to bind the APC/C, specific APC/C subunits must be phosphorylated by Cdk1 (among other Cdks). Therefore, when cdk activity is high in mitosis, and the cell must prepare to enter anaphase and exit mitosis, the APC/CCdc20 complex is activated. Once active, APC/CCdc20 promotes the degradation of Cdks by inactivating S/M cyclins. Cdk degradation brings about lower rates of APC/C phosphorylation and thus lower rates of CDC20 binding. In this way, the APC/CCdc20 complex inactivates itself by the end of mitosis.[34] However, because the cell does not immediately enter the cell cycle, Cdks can not immediately be reactivated. Multiple different mechanisms inhibit Cdks in G1: Cdk inhibitor proteins are expressed, and cyclin gene expression is down-regulated. Importantly, cyclin accumulation is also prevented by Cdh1.[34]
Cdh1
[edit]CDC20-homologue 1 (Cdh1) plays a complementary role to CDC20 in cell cycle progression. During the time of APC/CCdc20 activity, Cdh1 is phosphorylated and cannot bind to the APC/C. After metaphase, however, S/M-Cdks are inactivated by APC/CCdc20, and Cdh1 can exist in a non-phosphorylated state and bind the APC/C. This enables the APC/C to continue to degrade S/M cyclins (and thus S/M Cdks) until they are needed again in the next S-phase. How can S/M cyclins reappear to shepherd the cell into mitosis? The APC/CCdc20 does not recognize G1/S cyclins. Their concentration rises during G1, activating G1/S Cdks, which in turn phosphorylate Cdh1 and gradually relieve the inhibition on S/M cyclins.[34]
Spindle assembly checkpoint
[edit]CDC20 is also a part of, and regulated by, the spindle assembly checkpoint (SAC). This checkpoint ensures that anaphase proceeds only when the centromeres of all sister chromatids lined up on the metaphase plate are properly attached to microtubules. The checkpoint is held active by any unattached centromere; only when all centromeres are attached will anaphase commence. The APC/CCdc20 is an important target of the SAC, which consists of several different proteins, including Mad2, Mad3(BubR1), and Bub3. In fact, these three proteins, together with CDC20, likely form the mitotic checkpoint complex (MCC), which inhibits APC/CCdc20 so that anaphase cannot begin prematurely. Moreover, Bub1 phosphorylates and thus inhibits CDC20 directly, while in yeast Mad2 and Mad3, when bound to CDC20, trigger its autoubiquitination.[35]
Cancer
[edit]CDC20 is often elevated in cancerous tissues for multiple kinds of cancer. It is correlated with aggressiveness in breast cancer: higher levels are associated with poorer outcomes. CDC20 overexpression has also been reported in lung, gastric, and pancreatic cancers. For gastric and pancreatic cancers, higher levels are correlated with tumor size, histological grade (the abnormality of the cells), and metastases to the lymph nodes. In colorectal cancer and non-small-cell lung carcinoma, it is associated with cancer stage, and thus has been proposed as a biomarker to help predict the prognosis for people with either cancer.[36]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000117399 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000006398 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Weinstein J, Jacobsen FW, Hsu-Chen J, Wu T, Baum LG (May 1994). "A novel mammalian protein, p55CDC, present in dividing cells is associated with protein kinase activity and has homology to the Saccharomyces cerevisiae cell division cycle proteins Cdc20 and Cdc4". Mol Cell Biol. 14 (5): 3350–63. doi:10.1128/MCB.14.5.3350. PMC 358701. PMID 7513050.
- ^ Weinstein J (December 1997). "Cell cycle-regulated expression, phosphorylation, and degradation of p55Cdc. A mammalian homolog of CDC20/Fizzy/slp1". J Biol Chem. 272 (45): 28501–11. doi:10.1074/jbc.272.45.28501. PMID 9353311.
- ^ "Entrez Gene: CDC20 cell division cycle 20 homolog (S. cerevisiae)".
- ^ Hartwell LH, Culotti J, Reid B (June 1970). "Genetic Control of the Cell-Division Cycle in Yeast, I. Detection of Mutants". Proc. Natl. Acad. Sci. U.S.A. 66 (2): 352–9. Bibcode:1970PNAS...66..352H. doi:10.1073/pnas.66.2.352. PMC 283051. PMID 5271168.
- ^ Hartwell LH, Mortimer RK, Culotti J, Culotti M (June 1973). "Genetic Control of the Cell Division Cycle in Yeast: V. Genetic Analysis of cdc Mutants". Genetics. 74 (2): 267–286. doi:10.1093/genetics/74.2.267. PMC 1212945. PMID 17248617.
- ^ King RW, Peters JM, Tugendreich S, Rolfe M, Hieter P, Kirschner MW (April 1995). "A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B". Cell. 81 (2): 279–88. doi:10.1016/0092-8674(95)90338-0. PMID 7736580. S2CID 16958690.
- ^ Sudakin V, Ganoth D, Dahan A, Heller H, Hershko J, Luca FC, Ruderman JV, Hershko A (February 1995). "The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis". Mol. Biol. Cell. 6 (2): 185–97. doi:10.1091/mbc.6.2.185. PMC 275828. PMID 7787245.
- ^ a b c Vodermaier HC, Gieffers C, Maurer-Stroh S, Eisenhaber F, Peters JM (September 2003). "TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1". Curr. Biol. 13 (17): 1459–68. Bibcode:2003CBio...13.1459V. doi:10.1016/S0960-9822(03)00581-5. ISSN 0960-9822. PMID 12956947. S2CID 5942532.
- ^ a b c d e Nilsson J, Yekezare M, Minshull J, Pines J (December 2008). "The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction". Nat. Cell Biol. 10 (12): 1411–20. doi:10.1038/ncb1799. PMC 2635557. PMID 18997788.
- ^ Fang G (March 2002). "Checkpoint Protein BubR1 Acts Synergistically with Mad2 to Inhibit Anaphase-promoting Complex". Mol. Biol. Cell. 13 (3): 755–66. doi:10.1091/mbc.01-09-0437. ISSN 1059-1524. PMC 99596. PMID 11907259.
- ^ Wu H, Lan Z, Li W, Wu S, Weinstein J, Sakamoto KM, Dai W (September 2000). "p55CDC/hCDC20 is associated with BUBR1 and may be a downstream target of the spindle checkpoint kinase". Oncogene. 19 (40): 4557–62. doi:10.1038/sj.onc.1203803. ISSN 0950-9232. PMID 11030144. S2CID 23544995.
- ^ a b c d Kallio MJ, Beardmore VA, Weinstein J, Gorbsky GJ (September 2002). "Rapid microtubule-independent dynamics of Cdc20 at kinetochores and centrosomes in mammalian cells". J. Cell Biol. 158 (5): 841–7. doi:10.1083/jcb.200201135. ISSN 0021-9525. PMC 2173153. PMID 12196507.
- ^ Sudakin V, Chan GK, Yen TJ (September 2001). "Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2". J. Cell Biol. 154 (5): 925–36. doi:10.1083/jcb.200102093. ISSN 0021-9525. PMC 2196190. PMID 11535616.
- ^ a b Skoufias DA, Andreassen PR, Lacroix FB, Wilson L, Margolis RL (April 2001). "Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints". Proc. Natl. Acad. Sci. U.S.A. 98 (8): 4492–7. Bibcode:2001PNAS...98.4492S. doi:10.1073/pnas.081076898. PMC 31862. PMID 11274370.
- ^ a b c Kallio M, Weinstein J, Daum JR, Burke DJ, Gorbsky GJ (June 1998). "Mammalian p55CDC Mediates Association of the Spindle Checkpoint Protein Mad2 with the Cyclosome/Anaphase-promoting Complex, and is Involved in Regulating Anaphase Onset and Late Mitotic Events". J. Cell Biol. 141 (6): 1393–406. doi:10.1083/jcb.141.6.1393. ISSN 0021-9525. PMC 2132789. PMID 9628895.
- ^ a b D'Angiolella V, Mari C, Nocera D, Rametti L, Grieco D (October 2003). "The spindle checkpoint requires cyclin-dependent kinase activity". Genes Dev. 17 (20): 2520–5. doi:10.1101/gad.267603. ISSN 0890-9369. PMC 218146. PMID 14561775.
- ^ a b Wassmann K, Benezra R (September 1998). "Mad2 transiently associates with an APC/p55Cdc complex during mitosis". Proc. Natl. Acad. Sci. U.S.A. 95 (19): 11193–8. Bibcode:1998PNAS...9511193W. doi:10.1073/pnas.95.19.11193. ISSN 0027-8424. PMC 21618. PMID 9736712.
- ^ Kramer ER, Gieffers C, Hölzl G, Hengstschläger M, Peters JM (November 1998). "Activation of the human anaphase-promoting complex by proteins of the CDC20/Fizzy family". Curr. Biol. 8 (22): 1207–10. Bibcode:1998CBio....8.1207K. doi:10.1016/S0960-9822(07)00510-6. ISSN 0960-9822. PMID 9811605. S2CID 17181162.
- ^ Ohtoshi A, Maeda T, Higashi H, Ashizawa S, Hatakeyama M (February 2000). "Human p55(CDC)/Cdc20 associates with cyclin A and is phosphorylated by the cyclin A-Cdk2 complex". Biochem. Biophys. Res. Commun. 268 (2): 530–4. doi:10.1006/bbrc.2000.2167. ISSN 0006-291X. PMID 10679238.
- ^ Hsu JY, Reimann JD, Sørensen CS, Lukas J, Jackson PK (May 2002). "E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC/C(Cdh1)". Nat. Cell Biol. 4 (5): 358–66. doi:10.1038/ncb785. ISSN 1465-7392. PMID 11988738. S2CID 25403043.
- ^ a b Yoon YM, Baek KH, Jeong SJ, Shin HJ, Ha GH, Jeon AH, Hwang SG, Chun JS, Lee CW (September 2004). "WD repeat-containing mitotic checkpoint proteins act as transcriptional repressors during interphase". FEBS Lett. 575 (1–3): 23–9. doi:10.1016/j.febslet.2004.07.089. ISSN 0014-5793. PMID 15388328. S2CID 21762011.
- ^ Zhang Y, Lees E (August 2001). "Identification of an Overlapping Binding Domain on Cdc20 for Mad2 and Anaphase-Promoting Complex: Model for Spindle Checkpoint Regulation". Mol. Cell. Biol. 21 (15): 5190–9. doi:10.1128/MCB.21.15.5190-5199.2001. ISSN 0270-7306. PMC 87243. PMID 11438673.
- ^ Sihn CR, Suh EJ, Lee KH, Kim TY, Kim SH (November 2003). "p55CDC/hCDC20 mutant induces mitotic catastrophe by inhibiting the MAD2-dependent spindle checkpoint activity in tumor cells". Cancer Lett. 201 (2): 203–10. doi:10.1016/S0304-3835(03)00465-8. ISSN 0304-3835. PMID 14607335.
- ^ Luo X, Fang G, Coldiron M, Lin Y, Yu H, Kirschner MW, Wagner G (March 2000). "Structure of the Mad2 spindle assembly checkpoint protein and its interaction with Cdc20". Nat. Struct. Biol. 7 (3): 224–9. doi:10.1038/73338. ISSN 1072-8368. PMID 10700282. S2CID 1721494.
- ^ Sironi L, Melixetian M, Faretta M, Prosperini E, Helin K, Musacchio A (November 2001). "Mad2 binding to Mad1 and Cdc20, rather than oligomerization, is required for the spindle checkpoint". EMBO J. 20 (22): 6371–82. doi:10.1093/emboj/20.22.6371. ISSN 0261-4189. PMC 125308. PMID 11707408.
- ^ Fang G, Yu H, Kirschner MW (June 1998). "The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation". Genes Dev. 12 (12): 1871–83. doi:10.1101/gad.12.12.1871. ISSN 0890-9369. PMC 316912. PMID 9637688.
- ^ Privette LM, Weier JF, Nguyen HN, Yu X, Petty EM (July 2008). "Loss of CHFR in Human Mammary Epithelial Cells Causes Genomic Instability by Disrupting the Mitotic Spindle Assembly Checkpoint". Neoplasia. 10 (7): 643–52. doi:10.1593/neo.08176. PMC 2435002. PMID 18592005.
- ^ Vodermaier HC (October 2001). "Cell cycle: Waiters serving the Destruction machinery". Curr. Biol. 11 (20): R834–7. Bibcode:2001CBio...11.R834V. doi:10.1016/S0960-9822(01)00498-5. PMID 11676939. S2CID 11277828.
- ^ Kraft C, Vodermaier HC, Maurer-Stroh S, Eisenhaber F, Peters JM (May 2005). "The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates". Mol. Cell. 18 (5): 543–53. doi:10.1016/j.molcel.2005.04.023. PMID 15916961.
- ^ a b c Morgan DL (2007). The cell cycle: principles of control. London: Published by New Science Press in association with Oxford University Press. ISBN 978-0-87893-508-6.
- ^ Yu H (July 2007). "Cdc20: a WD40 activator for a cell cycle degradation machine". Mol. Cell. 27 (1): 3–16. doi:10.1016/j.molcel.2007.06.009. PMID 17612486.
- ^ Curtis NL, Ruda GF, Brennan P, Bolanos-Garcia VM (2020). "Deregulation of Chromosome Segregation and Cancer". Annual Review of Cancer Biology. 4: 257–278. doi:10.1146/annurev-cancerbio-030419-033541.
Further reading
[edit]- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Kallio M, Weinstein J, Daum JR, Burke DJ, Gorbsky GJ (1998). "Mammalian p55CDC Mediates Association of the Spindle Checkpoint Protein Mad2 with the Cyclosome/Anaphase-promoting Complex, and is Involved in Regulating Anaphase Onset and Late Mitotic Events". J. Cell Biol. 141 (6): 1393–406. doi:10.1083/jcb.141.6.1393. PMC 2132789. PMID 9628895.
- Fang G, Yu H, Kirschner MW (1998). "The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation". Genes Dev. 12 (12): 1871–83. doi:10.1101/gad.12.12.1871. PMC 316912. PMID 9637688.
- Weinstein J, Karim J, Geschwind DH, Nelson SF, Krumm J, Sakamoto KM (1998). "Genomic organization, 5' flanking enhancer region, and chromosomal assignment of the cell cycle gene, p55Cdc". Mol. Genet. Metab. 64 (1): 52–7. doi:10.1006/mgme.1998.2698. PMID 9682218.
- Fang G, Yu H, Kirschner MW (1998). "Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1". Mol. Cell. 2 (2): 163–71. doi:10.1016/S1097-2765(00)80126-4. PMID 9734353.
- Wassmann K, Benezra R (1998). "Mad2 transiently associates with an APC/p55Cdc complex during mitosis". Proc. Natl. Acad. Sci. U.S.A. 95 (19): 11193–8. Bibcode:1998PNAS...9511193W. doi:10.1073/pnas.95.19.11193. PMC 21618. PMID 9736712.
- Kramer ER, Gieffers C, Hölzl G, Hengstschläger M, Peters JM (1999). "Activation of the human anaphase-promoting complex by proteins of the CDC20/Fizzy family". Curr. Biol. 8 (22): 1207–10. Bibcode:1998CBio....8.1207K. doi:10.1016/S0960-9822(07)00510-6. PMID 9811605. S2CID 17181162.
- Cahill DP, da Costa LT, Carson-Walter EB, Kinzler KW, Vogelstein B, Lengauer C (1999). "Characterization of MAD2B and other mitotic spindle checkpoint genes". Genomics. 58 (2): 181–7. doi:10.1006/geno.1999.5831. PMID 10366450.
- Farruggio DC, Townsley FM, Ruderman JV (1999). "Cdc20 associates with the kinase aurora2/Aik". Proc. Natl. Acad. Sci. U.S.A. 96 (13): 7306–11. Bibcode:1999PNAS...96.7306F. doi:10.1073/pnas.96.13.7306. PMC 22081. PMID 10377410.
- Ohtoshi A, Maeda T, Higashi H, Ashizawa S, Hatakeyama M (2000). "Human p55(CDC)/Cdc20 associates with cyclin A and is phosphorylated by the cyclin A-Cdk2 complex". Biochem. Biophys. Res. Commun. 268 (2): 530–4. doi:10.1006/bbrc.2000.2167. PMID 10679238.
- Luo X, Fang G, Coldiron M, Lin Y, Yu H, Kirschner MW, Wagner G (2000). "Structure of the Mad2 spindle assembly checkpoint protein and its interaction with Cdc20". Nat. Struct. Biol. 7 (3): 224–9. doi:10.1038/73338. PMID 10700282. S2CID 1721494.
- Wu H, Lan Z, Li W, Wu S, Weinstein J, Sakamoto KM, Dai W (2000). "p55CDC/hCDC20 is associated with BUBR1 and may be a downstream target of the spindle checkpoint kinase". Oncogene. 19 (40): 4557–62. doi:10.1038/sj.onc.1203803. PMID 11030144. S2CID 23544995.
- Skoufias DA, Andreassen PR, Lacroix FB, Wilson L, Margolis RL (2001). "Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints". Proc. Natl. Acad. Sci. U.S.A. 98 (8): 4492–7. Bibcode:2001PNAS...98.4492S. doi:10.1073/pnas.081076898. PMC 31862. PMID 11274370.
- Reimann JD, Freed E, Hsu JY, Kramer ER, Peters JM, Jackson PK (2001). "Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex". Cell. 105 (5): 645–55. doi:10.1016/S0092-8674(01)00361-0. PMID 11389834. S2CID 16366514.
- Zhang Y, Lees E (2001). "Identification of an Overlapping Binding Domain on Cdc20 for Mad2 and Anaphase-Promoting Complex: Model for Spindle Checkpoint Regulation". Mol. Cell. Biol. 21 (15): 5190–9. doi:10.1128/MCB.21.15.5190-5199.2001. PMC 87243. PMID 11438673.
- Chen J, Fang G (2001). "MAD2B is an inhibitor of the anaphase-promoting complex". Genes Dev. 15 (14): 1765–70. doi:10.1101/gad.898701. PMC 312737. PMID 11459826.
External links
[edit]- Human CDC20 genome location and CDC20 gene details page in the UCSC Genome Browser.