Grey columns
| Grey columns | |
|---|---|
 Cross section of the spinal cord. The three grey columns make up the butterfly-shaped shaded region | |
| Details | |
| Identifiers | |
| Latin | columnae griseae |
| TA98 | A14.1.02.101 |
| TA2 | 6063 |
| FMA | 77867 |
| Anatomical terminology | |
The grey columns are three regions of the somewhat ridge-shaped mass of grey matter in the spinal cord.[1] These regions present as three columns: the anterior grey column, the posterior grey column, and the lateral grey column, all of which are visible in cross-section of the spinal cord.
The anterior grey column is made up of alpha motor neurons, gamma motor neurons, and small neurons thought to be interneurons.[2] It affects the skeletal muscles.
The posterior grey column receives several types of sensory information regarding touch and sensation from receptors in the skin, bones, and joints, including fine touch, proprioception, and vibration.[citation needed] It contains the cell bodies of second-order sensory neurons and their synapses with the pseudounipolar first-order sensory neurons (whose cell bodies are located within the sensory ganglia (a.k.a. dorsal root ganglia)).
The lateral grey column is only present in the thoracic region and upper lumbar segments (T1-L2). It contains preganglionic cell bodies of the autonomic nervous system and sensory relay neurons.
Structure
[edit]Anterior grey column
[edit]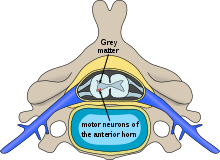
The anterior grey column, (also known as the anterior horn of spinal cord and anterior cornu) is broad and of a rounded or quadrangular shape. Its posterior part is termed the base, and its anterior part the head, but these are not differentiated from each other by any well-defined constriction. It is separated from the surface of the spinal cord by a layer of white substance which is traversed by the bundles of the anterior nerve roots. In the thoracic region, the posterolateral part of the anterior column projects laterally as a triangular field, which is named the lateral grey column. It comprises three different types of neurons, two types of lower motor neuron – large alpha motor neurons, and medium gamma motor neurons, and small neurons thought to be interneurons.[2] These neurons differ in both their morphology and in their patterns of connectivity.[3] They are organized in the same manner as the muscles they innervate.[4]
Alpha motor neurons
[edit]Alpha motor neurons are lower motor neurons that innervate extrafusal muscle fibers to generate force at neuromuscular junctions at the start of a muscle contraction. They have large cell bodies and receive proprioceptive input.[3] They have been shown to reduce in population, but not in size with age.[2] Damage to these cell bodies can lead to severe muscle weakness and loss of reflexes, and is also associated with ALS.[5][6]
Gamma motor neurons
[edit]Gamma motor neurons innervate intrafusal muscle fibers that control the sensitivity of muscle spindles to stretch. They have smaller cell bodies than alpha motor neurons and do not receive proprioceptive input.[3] They have been shown to reduce in numbers but not size with age.[2]
Small neurons
[edit]The physiology of the small neurons in the anterior column is not well understood. Their effects can be both excitatory and inhibitory. They are suspected to be interneurons and have been shown to reduce in size but not numbers with age.[2]
Clinical significance
It is these cells that are affected in the following diseases,[citation needed] – amyotrophic lateral sclerosis, spinal and bulbar muscular atrophy, Charcot–Marie–Tooth disease, progressive muscular atrophy, all spinal muscular atrophies, poliomyelitis, and West Nile virus.
Pharmacological interaction
The anterior grey column is the target for some spasmolytic medications. Norepinephrine release here, (as induced by cyclobenzaprine) reduces spasms by innervation (reducing nerve activity) of alpha motor neurons via interaction with gamma fibers.[7]
Posterior grey column
[edit]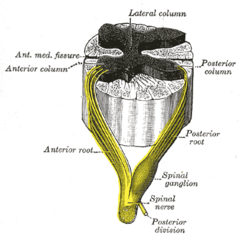
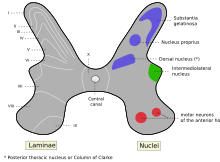
The posterior grey column, also known as the posterior (or dorsal) horn of spinal cord, is subdivided into six layers known as Rexed laminae, based on the type of sensory information sent to each section.[8]
- Marginal nucleus of spinal cord (lamina I)
- Substantia gelatinosa of Rolando (lamina II)
- Nucleus proprius (laminae III, IV)
- Spinal lamina V, the neck of the posterior horn [9]
- Spinal lamina VI, the base of the posterior horn.
The other four laminae are located in the other two grey columns in the spinal cord.
The function of the spinal dorsal horn is to process and integrate sensory information from the peripheral nervous system. It receives inputs from primary afferent fibers and modulatory systems, and it projects to higher brain centers and motor neurons. The dorsal horn circuitry is involved in various aspects of sensory processing, including discrimination, integration, and modulation of nociceptive and non-nociceptive signals. Dysfunction of the dorsal horn circuitry has been implicated in chronic pain conditions and other neurological disorders.[10]
Laminae I and II receive information from afferent neurons that sense nociception, temperature, and itching, laminae III and IV are sent information from neurons that sense mechanical pressure, and laminae V and VI are sent information from proprioceptors.[11] It is known to be the primary relay point for haptic and nociceptive messages.[12] The posterior horn is also known as a partially layered structure because only laminae I and II are well defined.
The column can also be separated by nociceptive and non-nociceptive senses. Laminae I and II are important in nociception, laminae III and IV are not involved nociception, and lamina V is involved in both nociception and non-nociception.[13]
The function of the spinal dorsal horn is to process and integrate sensory information from the peripheral nervous system. It receives inputs from primary afferent fibers and modulatory systems, and it projects to higher brain centers and motor neurons. The dorsal horn circuitry is involved in various aspects of sensory processing, including discrimination, integration, and modulation of nociceptive and non-nociceptive signals. Dysfunction of the dorsal horn circuitry has been implicated in chronic pain conditions and other neurological disorders.
Lamina I
[edit]Lamina I is also known as the marginal nucleus of spinal cord. The majority of posterior column projection neurons are located in lamina I, however most neurons in this layer are interneurons.[14] The main areas these neurons innervate are the caudal ventrolateral medulla (CVLM), the nucleus of the solitary tract (NTS), the lateral parabrachial area (LPb), the periaqueductal grey matter (PAG), and certain regions in the thalamus.[12] The CVLM receives nociceptive and cardiovascular responses.[15] The NTS receives cardio-respiratory inputs and affects reflex tachycardia from noxious stimulation.[16] The LPb projects to the amygdala and hypothalamus and is involved in the emotional response to pain.[17] The PAG develops ways to deal with pain and is a main target of analgesics. It projects to other parts of the brainstem.[18] The nuclei of the thalamus affect sensory and motivational aspects of pain.[19] The neurons of this lamina can be distinguished by their morphology as pyramidal, spindle, or multipolar.[20]
Lamina II
[edit]This layer is also known as the substantia gelatinosa of Rolando and has the highest density of neurons.[21] These neurons mediate the activity of nociceptive and temperature afferent fibers.[4] It is almost entirely made up of interneurons which can be further divided by their morphology. The four main morphological classes, based on the shape of their dendritic structure, are islet, central, vertical, and radial cells. The interneurons can also be divided by their function: excitatory or inhibitory. The excitatory interneurons release glutamate as their main neurotransmitter and the inhibitory interneurons use GABA and/or glycine as their main neurotransmitter. The neurons of this layer are only C fibers and contain almost no myelin.[22]
Laminae III and IV
[edit]These laminae are also known as the nucleus proprius and contain a much smaller density of neurons than lamina II.[21] There are projection neurons scattered throughout these layers.[14] Mechanosensitive A beta fibers terminate in these layers.[13] The layers receive input from lamina II and also control pain, temperature, and crude touch.[4] C fibers that control nociception and temperature and sensory information from mechanoreceptors are relayed here.[23]
Lamina V
[edit]This lamina is also known as the neck of the posterior column and receives information from mechanoreceptors and danger information from nociceptors.[23] It has different neurons in different regions. In the medial region it contains medium-sized triangular neurons and the lateral region contains medium-sized multipolar neurons.[21]
Lamina VI
[edit]This lamina is only found in the cervical and lumbar regions of the spinal cord. It receives afferent input from muscle fibers and joints.[4]
Lateral grey column
[edit]The lateral grey column, or the lateral horn of spinal cord, is part of the sympathetic nervous system and receives input from brain stem, organs, and hypothalamus. The lateral column is only present in the thoracic region and upper lumbar segments. The lateral grey column contains preganglionic cell bodies of the autonomic nervous system and sensory relay neurons.
Clinical significance
[edit]Neurons in the anterior column have been shown to be affected by amyotrophic lateral sclerosis (ALS). The number of large alpha motor neurons and medium gamma motor neurons was greatly reduced and the number of small neurons was either slightly or greatly reduced depending on the type of ALS.[24]

Muscular atrophy has also been shown to have an effect on neurons of the anterior column. A large loss of large alpha motor neurons, medium gamma motor neurons, and small neurons was recorded in cases of muscular atrophy.[25]
Damage to the lateral column can result in Horner's syndrome.
Multiple system atrophy (MSA), has also been linked to the lateral grey column. MSA has been shown to reduce the cell count in the lateral column by over 50%.
The posterior column has a prominent role in the pain system, it is the first central relay in the nociceptive pathway. The first-order afferent neuron carries sensory information to the second order neuron in the dorsal horn. The axon of the second order neuron, if it is a projection neuron and not an interneuron, then goes to the third order neuron in the thalamus. The thalamus is known as the "gateway to the cortex". The third order neuron then goes to the cerebral cortex. The afferent neurons are either A fibers or C fibers. A fibers are myelinated allowing for faster signal conduction. Among these there are A beta fibers which are faster and carry information about non-painful touch and A delta fibers which are slower and thinner than the A beta fibers. The C fibers are not myelinated and therefore slower.[14] C fibers that carry nociceptive signals can be divided into two types: fibers that contain neuropeptides, like substance P, and fibers that do not contain neuropeptides.[26] The two types terminate in very different areas. Non-peptidergic C fibers are linked to the skin, where they innervate the epidermis while peptidergic C fibers innervate other tissues and deeper parts of the skin.[14]
There are two main types of nociceptive signals: sensory and affective.
Sensory
[edit]Sensory nociceptive signals provide information about what kind of stimulus (heat, mechanical, etc.) is affecting the body and also indicates where on the body the stimulus is. Sensory nociceptive neurons have a small receptive field to help pinpoint the exact location of a stimulus.[27]
Affective
[edit]Affective nociceptive signals affect emotions. These signals go to the limbic system and tell the body to react to the danger stimulus (i.e. removing a hand from a hot stove). These neurons have larger receptive fields because the emotional reaction to most pain stimuli is similar.[27]
See also
[edit]- Beta motor neuron
- Gamma motor neuron
- Posterior column-medial lemniscus pathway
- Posterior horn of lateral ventricles
- Type Ia sensory fiber
References
[edit]![]() This article incorporates text in the public domain from page 753 of the 20th edition of Gray's Anatomy (1918)
This article incorporates text in the public domain from page 753 of the 20th edition of Gray's Anatomy (1918)
- ^ Henry Gray; Susan Standring; Harold Ellis; B. K. B. Berkovitz (2005), Gray's anatomy, p. 255
- ^ a b c d e Terao S, Sobue G, Hashizume Y, Li M, Inagaki T, Mitsuma T (Aug 1996). "Age-related changes in human spinal ventral horn cells with special reference to the loss of small neurons in the intermediate zone: a quantitative analysis". Acta Neuropathologica. 92 (2): 109–14. doi:10.1007/s004010050497. PMID 8841655. S2CID 19467756.
- ^ a b c Friese A, Kaltschmidt JA, Ladle DR, Sigrist M, Jessell TM, Arber S (Aug 11, 2009). "Gamma and alpha motor neurons distinguished by expression of transcription factor Err3". Proceedings of the National Academy of Sciences of the United States of America. 106 (32): 13588–13593. Bibcode:2009PNAS..10613588F. doi:10.1073/pnas.0906809106. PMC 2716387. PMID 19651609.
- ^ a b c d Siegel, Allan (2010). Essential Neuroscience. Lippincott Williams & Wilkins. ISBN 978-0781783835.
- ^ Haines, Duane (2012). Fundamental Neuroscience for Basic and Clinical Applications. Saunders. p. 138. ISBN 978-1437702941.
- ^ Van Den Berg-Vos, RM; Van Den Berg, LH; Visser, J; de Visser, M; Franssen, H; Wokke, JH (November 2003). "The spectrum of lower motor neuron syndromes". Journal of Neurology. 250 (11): 1279–92. doi:10.1007/s00415-003-0235-9. PMID 14648143.
- ^ "Cyclobenzaprine". DrugBank.
- ^ Cagle, MC; Honig, MG (July 2013). "Parcellation of Cblns 1, 2, and 4 among different subpopulations of dorsal horn neurons in mouse spinal cord". Journal of Comparative Neurology. 522 (2): 479–97. doi:10.1002/cne.23422. PMC 3855892. PMID 23853053.
- ^ Woolsey, Robert M.; Vernon W. Lin; Cardenas, Diana D.; Cutter, Nancy C.; Frederick S. Frost; Margaret C. Hammond; Laurie B. Lindblom; Inder Perkash; Robert Waters (2002). Spinal Cord Medicine: Principles and Practice. Demos Medical Publishing. ISBN 1-888799-61-7.
- ^ Harding, Erika K.; Fung, Samuel Wanchi; Bonin, Robert P. (2020). "Insights Into Spinal Dorsal Horn Circuit Function and Dysfunction Using Optical Approaches". Frontiers in Neural Circuits. 14: 31. doi:10.3389/fncir.2020.00031. ISSN 1662-5110. PMC 7303281. PMID 32595458.
- ^ Brown, AG (1981). Organization in the Spinal Cord: The Anatomy and Physiology of Identified Neurones. Berlin: Springer-Verlag.
- ^ a b Gauriau, Caroline; Bernard, Jean-François (2004). "A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: The forebrain". The Journal of Comparative Neurology. 468 (1): 24–56. doi:10.1002/cne.10873. PMID 14648689. S2CID 26117604.
- ^ a b Kato G, Kawasaki Y, Koga K, Uta D, Kosugi M, Yasaka T, Yoshimura M, Ji RR, Strassman AM (April 2009). "Organization of intralaminar and translaminar neuronal connectivity in the superficial spinal dorsal horn". The Journal of Neuroscience. 29 (16): 5088–5099. doi:10.1523/JNEUROSCI.6175-08.2009. PMC 2777732. PMID 19386904.
- ^ a b c d Todd, Andrew (Dec 2010). "Neuronal circuitry for pain processing in the dorsal horn". Nature Reviews Neuroscience. 11 (12): 823–836. doi:10.1038/nrn2947. PMC 3277941. PMID 21068766.
- ^ Lima D, Albino-Teixeira A, Tavares I (Mar 2002). "The caudal medullary ventrolateral reticular formation in nociceptive-cardiovascular integration. An experimental study in the rat". Experimental Physiology. 87 (2): 267–74. doi:10.1113/eph8702354. PMID 11856973. S2CID 13605412.
- ^ Boscan P, Pickering AE, Paton JF (Mar 2002). "The nucleus of the solitary tract: an integrating station for nociceptive and cardiorespiratory afferents". Experimental Physiology. 87 (2): 259–66. doi:10.1113/eph8702353. PMID 11856972. S2CID 22373004.
- ^ Gauriau, C; Bernard, J. F. (Mar 2002). "Pain pathways and parabrachial circuits in the rat". Experimental Physiology. 87 (2): 251–8. doi:10.1113/eph8702357. PMID 11856971. S2CID 42574814.
- ^ Heinricher MM, Tavares I, Leith JL, Lumb BM (Apr 2009). "Descending control of nociception: Specificity, recruitment and plasticity". Brain Research Reviews. 60 (1): 214–225. doi:10.1016/j.brainresrev.2008.12.009. PMC 2894733. PMID 19146877.
- ^ Gauriau, C.; Bernard, J. F. (Jan 2004). "Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat". Journal of Neuroscience. 24 (3): 752–61. doi:10.1523/JNEUROSCI.3272-03.2004. PMC 6729251. PMID 14736861.
- ^ Han ZS, Zhang ET, Craig AD (Jul 1998). "Nociceptive and thermoreceptive lamina I neurons are anatomically distinct". Nature Neuroscience. 1 (3): 218–25. doi:10.1038/665. PMID 10195146. S2CID 21222047.
- ^ a b c Paxinos, George (2004). The Human Nervous System. Academic Press. ISBN 978-0125476263.
- ^ Grudt, T. J.; Perl, E. R. (Apr 1, 2002). "Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn". The Journal of Physiology. 540 (Pt 1): 189–207. doi:10.1113/jphysiol.2001.012890. PMC 2290200. PMID 11927679.
- ^ a b Muthayya, NM (2002). Human Physiology. New Delhi: Jaypee Brothers Medical Publishers.
- ^ Terao S, Sobue G, Hashizume Y, Mitsuma T, Takahashi A (Feb 1994). "Disease-specific patterns of neuronal loss in the spinal ventral horn in amyotrophic lateral sclerosis, multiple system atrophy and X-linked recessive bulbospinal neuronopathy, with special reference to the loss of small neurons in the intermediate zone". Journal of Neurology. 241 (4): 196–203. doi:10.1007/bf00863768. PMID 8195817. S2CID 23011881.
- ^ Terao S, Sobue G, Li M, Hashizume Y, Tanaka F, Mitsuma T (Jan 1997). "The lateral corticospinal tract and spinal ventral horn in X-linked recessive spinal and bulbar muscular atrophy: a quantitative study". Acta Neuropathologica. 93 (1): 1–6. doi:10.1007/s004010050575. PMID 9006650. S2CID 12023369.
- ^ Snider, W. D.; McMahon, S. B. (Apr 1998). "Tackling pain at the source: new ideas about nociceptors". Neuron. 20 (4): 629–32. doi:10.1016/s0896-6273(00)81003-x. PMID 9581756. S2CID 18001663.
- ^ a b Price, Donald (Oct 2002). "Central neural mechanisms that interrelate sensory and affective dimensions of pain". Molecular Interventions. 2 (6): 392–403, 339. doi:10.1124/mi.2.6.392. PMID 14993415.
