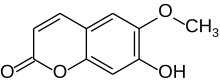
Scopoletin
| |
| Names | |
|---|---|
| Preferred IUPAC name
7-Hydroxy-6-methoxy-2H-1-benzopyran-2-one | |
| Other names
7-Hydroxy-6-methoxy-2H-chromen-2-one
7-Hydroxy-6-methoxychromen-2-one Gelseminic acid Chrysatropic acid Scopoletine 6-Methylesculetin Murrayetin Scopoletol Escopoletin Methylesculetin 6-O-Methylesculetin Esculetin-6-methyl ether 7-Hydroxy-5-methoxycoumarin 6-Methoxyumbelliferone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.001.975 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H8O4 | |
| Molar mass | 192.16 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Scopoletin is a coumarin found in the root of plants in the genus Scopolia such as Scopolia carniolica and Scopolia japonica, in chicory, in Artemisia scoparia, in the roots and leaves of stinging nettle (Urtica dioica), in the passion flower, in Brunfelsia, in Viburnum prunifolium, in Solanum nigrum,[1] in Datura metel,[2] in Mallotus resinosus,[3] or and in Kleinhovia hospita. It can also be found in fenugreek,[4] vinegar,[5][4] some whiskies or in dandelion coffee. A similar coumarin is scoparone. Scopoletin is highly fluorescent when dissolved in DMSO or water and is regularly used as a fluorimetric assay for the detection of hydrogen peroxide in conjunction with horseradish peroxidase. When oxidized, its fluorescence is strongly suppressed.
Chemistry
[edit]Biosynthesis
[edit]Like most phenylpropanoids, the biosynthetic precursor to scopoletin acid is 4-coumaroyl-CoA.[6] Scopoletin is derived from 1,2-benzopyrones[7] which is the core structure of coumarins formed through hydroxylation of cinnamates, trans/cis isomerization of the side chain, and lactonization.[8] And CYP98A (C3’H) are enzymes belonging to the cytochrome P450 family that catalyze the meta-hydroxylation of p-coumarate derivatives, an important step in the phenylpropanoid pathway.[9] For scopoletin, most of biosynthetic investigations are based on Arabidopsis thaliana.
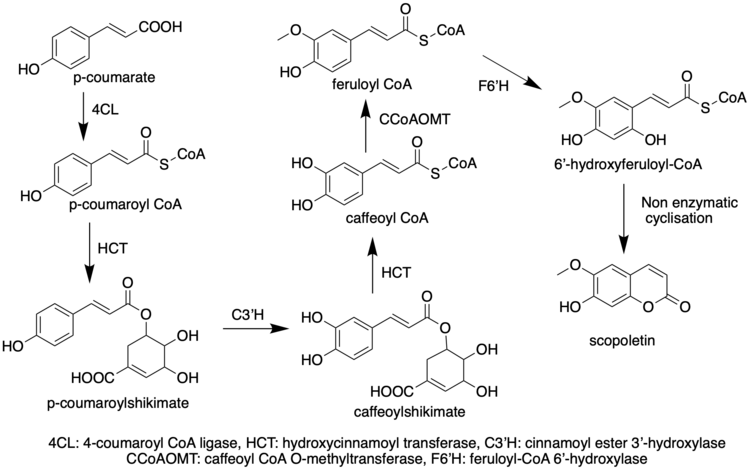
Derivatives/Related Compounds
[edit]Scopolin is a glucoside of scopoletin formed by the action of the enzyme scopoletin glucosyltransferase.
Uses
[edit]Traditional Medicine
[edit]It was usually used for rheumatic arthritis therapy in Traditional Chinese Medicine.
References
[edit]- ^ Zhao Y; Liu F; Lou HX (2010). "[Studies on the chemical constituents of Solanum nigrum]". Zhong Yao Cai (in Chinese). 33 (4): 555–556. PMID 20845784.
- ^ Han XL, Wang H, Zhang ZH, Tan Y, Wang JH (August 2015). "[Study on Chemical Constituents in Seeds of Datura metel from Xinjiang]". Zhong Yao Cai = Zhongyaocai = Journal of Chinese Medicinal Materials (in Chinese). 38 (8): 1646–8. PMID 26983236.
- ^ Ma J; Jones SH; Hecht SM (2004). "A coumarin from Mallotus resinosus that mediates DNA cleavage". J Nat Prod. 67 (9): 1614–1616. doi:10.1021/np040129c. PMID 15387675.
- ^ a b Ouzir, M; El Bairi, K; Amzazi, S (October 2016). "Toxicological properties of fenugreek (Trigonella foenum graecum)". Food and Chemical Toxicology. 96: 145–54. doi:10.1016/j.fct.2016.08.003. PMID 27498339.
- ^ Analysis of polyphenolic compounds of different vinegar samples. Miguel Carrero Gálvez, Carmelo García Barroso and Juan Antonio Pérez-Bustamante, Zeitschrift für Lebensmitteluntersuhung und -Forschung A, Volume 199, Number 1, pages 29-31, doi:10.1007/BF01192948
- ^ Vogt, T. (2010). "Phenylpropanoid Biosynthesis". Molecular Plant. 3: 2–20. doi:10.1093/mp/ssp106. PMID 20035037.
- ^ Beeching, John R.; Han, Yuanhuai; Gómez-Vásquez, Rocío; Day, Robert C.; Cooper, Richard M. (1998), "Wound and Defense Responses in Cassava as Related to Post-Harvest Physiological Deterioration", Phytochemical Signals and Plant—Microbe Interactions, Springer US, pp. 231–248, doi:10.1007/978-1-4615-5329-8_12, ISBN 9780306459177
- ^ Kai, Kosuke; Mizutani, Masaharu; Kawamura, Naohiro; Yamamoto, Ryotaro; Tamai, Michiko; Yamaguchi, Hikaru; Sakata, Kanzo; Shimizu, Bun-ichi (September 2008). "Scopoletin is biosynthesized viaortho-hydroxylation of feruloyl CoA by a 2-oxoglutarate-dependent dioxygenase inArabidopsis thaliana". The Plant Journal. 55 (6): 989–999. doi:10.1111/j.1365-313x.2008.03568.x. ISSN 0960-7412. PMID 18547395.
- ^ Schoch, Guillaume; Goepfert, Simon; Morant, Marc; Hehn, Alain; Meyer, Denise; Ullmann, Pascaline; Werck-Reichhart, Danièle (2001-06-27). "CYP98A3 fromArabidopsis thalianaIs a 3′-Hydroxylase of Phenolic Esters, a Missing Link in the Phenylpropanoid Pathway" (PDF). Journal of Biological Chemistry. 276 (39): 36566–36574. doi:10.1074/jbc.m104047200. ISSN 0021-9258. PMID 11429408. S2CID 11765327.
