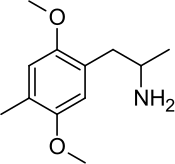
2,5-Dimethoxy-4-methylamphetamine
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 2,5-Dimethoxy-4-methylamphetamine; 4-Methyl-2,5-dimethoxyamphetamine; 2,5-Dimethoxy-4-methyl-α-methylphenethylamine; Des-oxy-methyl; DOM; DMMTA; α-Me-2C-D; STP; Serenity, Tranquility, and Peace; Super Terrific Psychedelic; Stop The Police; Too Stupid to Puke[1] |
| Drug class | Serotonergic psychedelic; Hallucinogen; Serotonin 5-HT2 receptor agonist |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H19NO2 |
| Molar mass | 209.289 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 61 °C (142 °F) |
| |
| |
| (verify) | |
2,5-Dimethoxy-4-methylamphetamine (DOM), also known as STP (standing for "Serenity, Tranquility, and Peace" and/or other phrases), is a psychedelic drug of the phenethylamine, amphetamine, and DOx families.[3][4][1][5][6] It is generally taken orally.[4][1][5]
DOM was first synthesized by Alexander Shulgin, and later described in his book PiHKAL: A Chemical Love Story (1991).[1] It is classified as a Schedule I controlled substance in the United States, and is similarly controlled in other parts of the world.[1] Internationally, it is a Schedule I drug under the Convention on Psychotropic Substances.[7]
Effects
[edit]Effects of this drug include substantial perceptual changes such as blurred vision, multiple images, vibration of objects, visual alterations, distorted shapes, enhancement of details, slowed passage of time, increased sexual drive and pleasure, and increased contrasts. It may cause mystical experiences and changes in consciousness. It may also cause pupillary dilation and a rise in systolic blood pressure.[8]
Side effects
[edit]Very little is known about the toxicity of DOM.
Pharmacology
[edit]Pharmacodynamics
[edit]Actions
[edit]| Target | Affinity (Ki, nM) |
|---|---|
| 5-HT1A | 3,656–14,200 (Ki) 12,800–13,900 (EC50) 54–74% (Emax) |
| 5-HT1B | >10,000 |
| 5-HT1D | 209 |
| 5-HT1E | 3,542 |
| 5-HT1F | ND |
| 5-HT2A | 2.1–507 (Ki) 1.1–40 (EC50) 103–132% (Emax) |
| 5-HT2B | 12–41 (Ki) 128–145 (EC50) 85% (Emax) |
| 5-HT2C | 19–3,980 (Ki) 0.23–22 (EC50) 94–119% (Emax) |
| 5-HT3 | >10,000 |
| 5-HT4 | ND |
| 5-HT5A | >10,000 |
| 5-HT6 | 8,155 |
| 5-HT7 | 1,591 |
| α1A | 3,219 |
| α1B | >10,000 |
| α1D | ND |
| α2A | 580 |
| α2B | 874 |
| α2C | 921 |
| β1 | >10,000 |
| β2 | 49 |
| D1–D5 | >10,000 |
| H1–H4 | >10,000 |
| M1, M2, M5 | >10,000 |
| M3, M4 | ND |
| TAAR1 | >10,000 (EC50) |
| I1 | >10,000 |
| σ1, σ2 | >10,000 |
| SERT | >100,000 (Ki) >100,000 (IC50) >100,000 (EC50) |
| NET | >100,000 (Ki) >70,000 (IC50) >100,000 (EC50) |
| DAT | >100,000 (Ki) 64,000 (IC50) >42,000 (EC50) |
| MAO-A | 24,000 (IC50) (rat) |
| MAO-B | >100,000 (IC50) (rat) |
| Notes: The smaller the value, the more avidly the drug binds to the site. All proteins are human unless otherwise specified. Refs: [9][10][11][12][13][14][15][16][17][18][19] | |
DOM acts as a selective 5-HT2A, 5-HT2B, and 5-HT2C receptor full agonist.[12][11][13] In one study, its affinities (Ki) were 12 nM for the serotonin 5-HT2A Its psychedelic effects are mediated by its agonistic properties at the 5-HT2A receptor. Due to its selectivity, DOM is often used in scientific research when studying the 5-HT2 receptor subfamily. DOM is a chiral molecule, and R-(−)-DOM is the more active enantiomer, functioning as a potent agonist of the serotonin 5-HT family of receptors, mainly of the 5-HT2 subtype.[20]
The drug is inactive as a human trace amine-associated receptor 1 (TAAR1) agonist but is an agonist of the rhesus monkey TAAR1.[15] DOM is inactive as a monoamine reuptake inhibitor and releasing agent.[14] It is a very weak monoamine oxidase inhibitor (MAOI), specifically of monoamine oxidase A (MAO-A), whereas it was inactive at monoamine oxidase B (MAO-B).[18][19]
Effects
[edit]In contrast to amphetamines like (–)-cathinone but similarly to mescaline, DOM has shown no stimulant-like or reinforcing effects in rhesus monkeys.[21][22][23][24] Conversely however, DOC has shown reinforcing effects, including conditioned place preference (CPP) and self-administration, in rodents similarly to methamphetamine.[25] This is analogous to other findings in which various 2C and NBOMe drugs have been found to produce dopaminergic elevations and reinforcing effects in rodents.[26][27][28][29][30][31][32]
Pharmacokinetics
[edit]According to Alexander Shulgin, the effects of DOM typically last 14 to 20 hours, though other clinical trials indicate a duration of 7 to 8 hours.[8]
Chemistry
[edit]DOM, also known as 2,5-dimethoxy-4-methylamphetamine or as 2,5-dimethoxy-4-methyl-α-methylphenethylamine, is a substituted phenethylamine and amphetamine and is a member of the DOx group of drugs.[3][4][5][6] It is structurally related to the naturally occurring phenethylamine psychedelic mescaline (3,4,5-trimethoxyphenethylamine).[6][33] Analogues of DOM include other DOx drugs such as DOET, DOB, DOI, DOC, and TMA, among others.[6] The α-desmethyl or phenethylamine analogue of DOM is 2C-D.[3][4] Ariadne is the α-ethyl or phenylisobutylamine analogue of DOM.[34][4]

The 2,6-dimethoxy positional isomer of DOM, known as Ψ-DOM, is also mentioned in PiHKAL as being active, as is the α-ethyl homologue Ariadne. Analogues where the methoxy groups at the 2,5- positions of the aromatic ring have been altered have also been synthesised and tested as part of an effort to identify the binding mode of DOM at the serotonin 5-HT2A receptor. Both the 2- and 5- O-desmethyl derivatives 2-DM-DOM and 5-DM-DOM, and the 2- and 5- ethyl analogues 2-Et-DOM and 5-Et-DOM, have been tested, but in all cases were significantly less potent than the corresponding methoxy compound, showing the importance of the oxygen lone pairs in 5-HT2A binding.[35][36]
History
[edit]STP was first synthesized and tested in 1963 by Alexander Shulgin, who was investigating the effect of 4-position substitutions on psychedelic amphetamines.[1][4]
In mid-1967, tablets containing 20 mg (later 10 mg) of STP were widely distributed in the Haight-Ashbury District of San Francisco under the name of STP, having been manufactured by underground chemists Owsley Stanley and Tim Scully.[1] This short-lived appearance of STP on the black market proved disastrous for several reasons.[1] First, the tablets contained an excessively high dose of the chemical.[1] This, combined with DOM's slow onset of action (which encouraged some users, familiar with drugs that have quicker onsets, such as LSD, to re-dose) and its remarkably long duration, caused many users to panic and sent some to the emergency room.[1] Second, treatment of such overdoses was complicated by the fact that no one at the time knew that the tablets called STP were, in fact, DOM, and there was no effective antidote.[1]
Society and culture
[edit]Legal status
[edit]Australia
[edit]DOM is schedule 9 under the Australia Poisons standard.[37] A schedule 9 substance is a "Substances which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities."[37]
Canada
[edit]Listed as a Schedule 1, as it is an analogue of amphetamine.
United Kingdom
[edit]DOM is a Class A drug in the United Kingdom under the Misuse of Drugs Act 1971.
United States
[edit]DOM is Schedule I in the United States. This means it is illegal to manufacture, buy, possess, or distribute (make, trade, own or give) without a DEA license.
References
[edit]- ^ a b c d e f g h i j k Baggott MJ (1 October 2023). "Learning about STP: A Forgotten Psychedelic from the Summer of Love" (PDF). History of Pharmacy and Pharmaceuticals. 65 (1): 93–116. doi:10.3368/hopp.65.1.93. ISSN 2694-3034. Retrieved 26 January 2025.
- ^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ^ a b c Shulgin A, Manning T, Daley PF (2011). "#60. DOM". The Shulgin Index, Volume One: Psychedelic Phenethylamines and Related Compounds. Vol. 1. Berkeley, CA: Transform Press. pp. 118–129. ISBN 978-0-9630096-3-0. OCLC 709667010.
- ^ a b c d e f Alexander T. Shulgin; Ann Shulgin (1991). "#68 DOM; STP; 2,5-DIMETHOXY-4-METHYLAMPHETAMINE". PiHKAL: A Chemical Love Story (1st ed.). Berkeley, CA: Transform Press. pp. 637–642. ISBN 978-0-9630096-0-9. OCLC 25627628.
- ^ a b c Wills B, Erickson T (9 March 2012). "Psychoactive Phenethylamine, Piperazine, and Pyrrolidinophenone Derivatives". In Barceloux DG (ed.). Medical Toxicology of Drug Abuse: Synthesized Chemicals and Psychoactive Plants. Wiley. pp. 156–192. doi:10.1002/9781118105955.ch10. ISBN 978-0-471-72760-6.
- ^ a b c d Shulgin AT (1978). "Psychotomimetic Drugs: Structure-Activity Relationships". In Iversen LL, Iversen SD, Snyder SH (eds.). Stimulants. Boston, MA: Springer US. pp. 243–333. doi:10.1007/978-1-4757-0510-2_6. ISBN 978-1-4757-0512-6.
- ^ "Green List: List of Psychotropic Substances Under International Control" (PDF) (23rd ed.). International Narcotics Control Board. August 2003. p. 4. Archived from the original (PDF) on 19 December 2013. Retrieved 22 February 2014.
- ^ a b Snyder, Solomon H.; Louis Faillace & Leo Hollister (3 November 1967). "2,5-Dimethoxy-4-methyl-amphetamine (STP): A New Hallucinogenic Drug" (PDF). Science. 158 (3801): 669–670. Bibcode:1967Sci...158..669S. doi:10.1126/science.158.3801.669. PMID 4860952. S2CID 24065654.
- ^ "PDSP Database". UNC (in Zulu). Retrieved 1 February 2025.
- ^ Liu, Tiqing. "BindingDB BDBM50005265 (+/-)2-(2,5-Dimethoxy-4-methyl-phenyl)-1-methyl-ethylamine::(-)2-(2,5-Dimethoxy-4-methyl-phenyl)-1-methyl-ethylamine::(Rec)2-(2,5-Dimethoxy-4-methyl-phenyl)-1-methyl-ethylamine; compound with 2-(2,5-dimethoxy-4-methyl-phenyl)-1-methyl-ethylamine::2-(2,5-Dimethoxy-4-methyl-phenyl)-1-methyl-ethylamine::2-(2,5-Dimethoxy-4-methyl-phenyl)-1-methyl-ethylamine((R)-(-)-DOM)::2-(2,5-Dimethoxy-4-methyl-phenyl)-1-methyl-ethylamine(DOM)::CHEMBL8600::DOM::DOM,R(-)::Racemic DOM". BindingDB. Retrieved 1 February 2025.
- ^ a b Ray TS (February 2010). "Psychedelics and the human receptorome". PLOS ONE. 5 (2): e9019. doi:10.1371/journal.pone.0009019. PMC 2814854. PMID 20126400.
- ^ a b Luethi D, Rudin D, Hoener MC, Liechti ME (2022). "Monoamine Receptor and Transporter Interaction Profiles of 4-Alkyl-Substituted 2,5-Dimethoxyamphetamines". The FASEB Journal. 36 (S1). doi:10.1096/fasebj.2022.36.S1.R2691. ISSN 0892-6638.
- ^ a b Eshleman AJ, Forster MJ, Wolfrum KM, Johnson RA, Janowsky A, Gatch MB (March 2014). "Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function". Psychopharmacology (Berl). 231 (5): 875–888. doi:10.1007/s00213-013-3303-6. PMC 3945162. PMID 24142203.
- ^ a b Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Johnson RA, Janowsky A (December 2018). "Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors". Biochem Pharmacol. 158: 27–34. doi:10.1016/j.bcp.2018.09.024. PMC 6298744. PMID 30261175.
- ^ a b Lewin AH, Miller GM, Gilmour B (December 2011). "Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class". Bioorg Med Chem. 19 (23): 7044–7048. doi:10.1016/j.bmc.2011.10.007. PMC 3236098. PMID 22037049.
- ^ Åstrand A, Guerrieri D, Vikingsson S, Kronstrand R, Green H (December 2020). "In vitro characterization of new psychoactive substances at the μ-opioid, CB1, 5HT1A, and 5-HT2A receptors-On-target receptor potency and efficacy, and off-target effects". Forensic Science International. 317: 110553. doi:10.1016/j.forsciint.2020.110553. PMID 33160102.
- ^ van Wijngaarden I, Soudijn W (1997). "5-HT2A, 5-HT2B and 5-HT2C receptor ligands". Pharmacochemistry Library. Vol. 27. Elsevier. pp. 161–197. doi:10.1016/s0165-7208(97)80013-x. ISBN 978-0-444-82041-9.
- ^ a b Reyes-Parada M, Iturriaga-Vasquez P, Cassels BK (2019). "Amphetamine Derivatives as Monoamine Oxidase Inhibitors". Front Pharmacol. 10: 1590. doi:10.3389/fphar.2019.01590. PMC 6989591. PMID 32038257.
- ^ a b Scorza MC, Carrau C, Silveira R, Zapata-Torres G, Cassels BK, Reyes-Parada M (December 1997). "Monoamine oxidase inhibitory properties of some methoxylated and alkylthio amphetamine derivatives: structure-activity relationships". Biochem Pharmacol. 54 (12): 1361–1369. doi:10.1016/s0006-2952(97)00405-x. PMID 9393679.
- ^ Sanders-Bush, E; Burris, KD; Knoth, K (September 1988). "Lysergic acid diethylamide and 2,5-dimethoxy-4-methylamphetamine are partial agonists at serotonin receptors linked to phosphoinositide hydrolysis". The Journal of Pharmacology and Experimental Therapeutics. 246 (3): 924–928. PMID 2843634.
- ^ Fantegrossi WE, Murnane KS, Reissig CJ (January 2008). "The behavioral pharmacology of hallucinogens". Biochem Pharmacol. 75 (1): 17–33. doi:10.1016/j.bcp.2007.07.018. PMC 2247373. PMID 17977517.
Despite the reasonably constant recreational use of hallucinogens since at least the early 1970s [44], the reinforcing effects of hallucinogens have not been widely investigated in laboratory animals. Indeed, one of the earliest studies on the reinforcing effects of drugs using the intravenous self-administration procedure in rhesus monkeys found that no animal initiated self-injection of mescaline either spontaneously or after one month of programmed administration [45]. Likewise, the phenethylamine hallucinogen 2,5-dimethoxy-4-methylamphetamine (DOM) was not effective in maintaining self-administration in rhesus monkeys [46]. Nevertheless, the hallucinogen-like phenethylamine 3,4-methylenedioxymethamphetamine (MDMA) has been shown to act as a reinforcer in intravenous self-administration paradigms in baboons [47], rhesus monkeys [48 – 50], rats [51] and mice [52].
- ^ Canal CE, Murnane KS (January 2017). "The serotonin 5-HT2C receptor and the non-addictive nature of classic hallucinogens". J Psychopharmacol. 31 (1): 127–143. doi:10.1177/0269881116677104. PMC 5445387. PMID 27903793.
One of the earliest studies on the reinforcing effects of drugs using the intravenous self-administration procedure in rhesus monkeys found that no animal initiated self-injection of mescaline either spontaneously or after one month of programmed administration, [...] (Deneau et al., 1969). The lack of mescaline self-administration stood in contrast to positive findings of self-administration of morphine, codeine, cocaine, amphetamine, pentobarbital, ethanol, and caffeine. A subsequent study with rhesus monkeys using 2,5-dimethoxy-4-methylamphetamine (DOM; Yanagita, 1986) provided similar results as the mescaline study. These findings have withstood the test of time, as the primary literature is virtually devoid of any accounts of self-administration of [classical hallucinogens (CH)], suggesting that there are very limited conditions under which laboratory animals voluntarily consume CH.
- ^ Yanagita T (June 1986). "Intravenous self-administration of (-)-cathinone and 2-amino-1-(2,5-dimethoxy-4-methyl)phenylpropane in rhesus monkeys". Drug Alcohol Depend. 17 (2–3): 135–141. doi:10.1016/0376-8716(86)90004-9. PMID 3743404.
- ^ Maguire DR (October 2024). "Evaluation of potential punishing effects of 2,5-dimethoxy-4-methylamphetamine (DOM) in rhesus monkeys responding under a choice procedure". Behav Pharmacol. 35 (7): 378–385. doi:10.1097/FBP.0000000000000787. PMID 39052019.
- ^ Cha HJ, Jeon SY, Jang HJ, Shin J, Kim YH, Suh SK (May 2018). "Rewarding and reinforcing effects of 4-chloro-2,5-dimethoxyamphetamine and AH-7921 in rodents". Neurosci Lett. 676: 66–70. doi:10.1016/j.neulet.2018.04.009. PMID 29626650.
- ^ Gil-Martins E, Barbosa DJ, Borges F, Remião F, Silva R (June 2025). "Toxicodynamic insights of 2C and NBOMe drugs - Is there abuse potential?". Toxicol Rep. 14: 101890. doi:10.1016/j.toxrep.2025.101890. PMID 39867514.
- ^ Kim YJ, Ma SX, Hur KH, Lee Y, Ko YH, Lee BR, Kim SK, Sung SJ, Kim KM, Kim HC, Lee SY, Jang CG (April 2021). "New designer phenethylamines 2C-C and 2C-P have abuse potential and induce neurotoxicity in rodents". Arch Toxicol. 95 (4): 1413–1429. Bibcode:2021ArTox..95.1413K. doi:10.1007/s00204-021-02980-x. PMID 33515270.
- ^ Custodio RJ, Sayson LV, Botanas CJ, Abiero A, You KY, Kim M, Lee HJ, Yoo SY, Lee KW, Lee YS, Seo JW, Ryu IS, Kim HJ, Cheong JH (November 2020). "25B-NBOMe, a novel N-2-methoxybenzyl-phenethylamine (NBOMe) derivative, may induce rewarding and reinforcing effects via a dopaminergic mechanism: Evidence of abuse potential". Addict Biol. 25 (6): e12850. doi:10.1111/adb.12850. PMID 31749223.
- ^ Seo JY, Hur KH, Ko YH, Kim K, Lee BR, Kim YJ, Kim SK, Kim SE, Lee YS, Kim HC, Lee SY, Jang CG (October 2019). "A novel designer drug, 25N-NBOMe, exhibits abuse potential via the dopaminergic system in rodents". Brain Res Bull. 152: 19–26. doi:10.1016/j.brainresbull.2019.07.002. PMID 31279579.
- ^ Jo C, Joo H, Youn DH, Kim JM, Hong YK, Lim NY, Kim KS, Park SJ, Choi SO (November 2022). "Rewarding and Reinforcing Effects of 25H-NBOMe in Rodents". Brain Sci. 12 (11): 1490. doi:10.3390/brainsci12111490. PMC 9688077. PMID 36358416.
- ^ Lee JG, Hur KH, Hwang SB, Lee S, Lee SY, Jang CG (August 2023). "Designer Drug, 25D-NBOMe, Has Reinforcing and Rewarding Effects through Change of a Dopaminergic Neurochemical System". ACS Chem Neurosci. 14 (15): 2658–2666. doi:10.1021/acschemneuro.3c00196. PMID 37463338.
- ^ Kim YJ, Kook WA, Ma SX, Lee BR, Ko YH, Kim SK, Lee Y, Lee JG, Lee S, Kim KM, Lee SY, Jang CG (April 2024). "The novel psychoactive substance 25E-NBOMe induces reward-related behaviors via dopamine D1 receptor signaling in male rodents". Arch Pharm Res. 47 (4): 360–376. doi:10.1007/s12272-024-01491-4. PMID 38551761.
- ^ Hassan Z, Bosch OG, Singh D, Narayanan S, Kasinather BV, Seifritz E, Kornhuber J, Quednow BB, Müller CP (2017). "Novel Psychoactive Substances-Recent Progress on Neuropharmacological Mechanisms of Action for Selected Drugs". Front Psychiatry. 8: 152. doi:10.3389/fpsyt.2017.00152. PMC 5563308. PMID 28868040.
The next, even though less accidental, producer of NPS hallucinogens was Alexander T. Shulgin, who synthesized hundreds of novel hallucinogenic tryptamines and phenylethylamines in his home laboratory. He described the synthesis of these compounds and also their psychotomimetic effects experienced in self-experiments in detail in his books PIHKAL and TIHKAL (199, 200). He created several dimethoxy-substituted phenylethylamines, such as DOM, 2,5-dimethoxy-4-bromoamphetamine (DOB), 2,5-dimethoxy-4-iodoamphetamine (DOI), and 2,5-dimethoxy-4-ethylamphetamine (DOET), which all display strong hallucinogenic properties. These drugs usually have much longer durations of action (12–30 h) and are much more potent agonists at 5-HT2A-Rs (50- to 175-fold) compared to their related phenylethylamine derivative mescaline (duration of action: 4–8 h) (189, 199, 200).
- ^ Cunningham MJ, Bock HA, Serrano IC, Bechand B, Vidyadhara DJ, Bonniwell EM, Lankri D, Duggan P, Nazarova AL, Cao AB, Calkins MM, Khirsariya P, Hwu C, Katritch V, Chandra SS, McCorvy JD, Sames D (January 2023). "Pharmacological Mechanism of the Non-hallucinogenic 5-HT2A Agonist Ariadne and Analogs". ACS Chemical Neuroscience. 14 (1): 119–135. doi:10.1021/acschemneuro.2c00597. PMC 10147382. PMID 36521179.
- ^ Eckler JR, Chang-Fong J, Rabin RA, Smith C, Teitler M, Glennon RA, Winter JC (July 2003). "Behavioral characterization of 2-O-desmethyl and 5-O-desmethyl metabolites of the phenylethylamine hallucinogen DOM". Pharmacology Biochemistry and Behavior. 75 (4): 845–852. doi:10.1016/S0091-3057(03)00159-X. PMID 12957227. S2CID 36463979.
- ^ Braden, Michael Robert (May 2007). Towards a Biophysical Basis of Hallucinogen Action (Thesis). Purdue University. OCLC 703618147. Retrieved 28 February 2012.
- ^ a b Poison Standard https://www.comlaw.gov.au/Details/F2015L01534/Html/Text#_Toc420496379 Archived 2015-12-22 at the Wayback Machine
