2,4-Dihydroxybenzoic acid
Appearance
(Redirected from 4-carboxyresorcinol)
| |
| Names | |
|---|---|
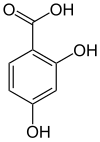
| Preferred IUPAC name
2,4-Dihydroxybenzoic acid | |
| Other names
β-Resorcylic acid
β-Resorcinolic acid p-Hydroxysalicylic acid 2,4-DHBA | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.770 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H6O4 | |
| Molar mass | 154.12 g/mol |
| Melting point | 229 °C (444 °F; 502 K)[2] |
| Acidity (pKa) | 3.11, 8.55, 14.0[1] |
| Hazards | |
| GHS labelling:[3] | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P280, P302+P352, P305+P351+P338 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2,4-Dihydroxybenzoic acid (β-resorcylic acid) is a dihydroxybenzoic acid.
As a resorcylic acid, it is one of the three isomeric crystalline acids that are both carboxyl derivatives of resorcinol and dihydroxy derivatives of benzoic acid.[4] Synthesis from resorcinol is via the Kolbe-Schmitt reaction.[5]
It is a degradation product of cyanidin glycosides from tart cherries in cell cultures.[6] It is also a metabolite found in human plasma after cranberry juice consumption.[7]
References
[edit]- ^ Haynes, p. 5.91
- ^ Haynes, p. 3.190
- ^ GHS: GESTIS 492493
- ^ Resorcyclic acid on merriam-webster on-line dictionary
- ^ Becker, Heinz G. O., ed. (1993). Organikum: organisch-chemisches Grundpraktikum (in German) (19th (rev. and expanded) ed.). Leipzig: Johann Ambrosius Barth. pp. 351–352. ISBN 978-3-335-00343-4.
- ^ Seeram, Navindra P.; Bourquin, Leslie D.; Nair, Muraleedharan G. (2001). "Degradation Products of Cyanidin Glycosides from Tart Cherries and Their Bioactivities". Journal of Agricultural and Food Chemistry. 49 (10): 4924–4929. doi:10.1021/jf0107508. PMID 11600045.
- ^ Zhang, Kai; Zuo, Yuegang (2004). "GC-MS Determination of Flavonoids and Phenolic and Benzoic Acids in Human Plasma after Consumption of Cranberry Juice". Journal of Agricultural and Food Chemistry. 52 (2): 222–227. doi:10.1021/jf035073r. PMID 14733499.
Cited sources
[edit]- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
