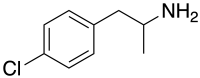
para-Chloroamphetamine
 | |
 | |
| Clinical data | |
|---|---|
| Other names | PCA; pCA; 4-Chloroamphetamine; 4-CA |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C9H12ClN |
| Molar mass | 169.65 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
para-Chloroamphetamine (PCA), also known as 4-chloroamphetamine (4-CA), is a substituted amphetamine and monoamine releaser similar to MDMA, but with substantially higher activity as a monoaminergic neurotoxin, thought to be due to the unrestrained release of both serotonin and dopamine by a metabolite.[1] It is used as a neurotoxin by neurobiologists to selectively kill serotonergic neurons for research purposes, in the same way that 6-hydroxydopamine is used to kill dopaminergic neurons.[2][3][4][5]
para-Chloroamphetamine has been detected as an apparent designer drug,[6] along with the related 3-chloroamphetamine, which is even more potent as a releaser of dopamine and serotonin but slightly less neurotoxic.[7][8][9][10][11]
The closely related N-methylated derivative, para-chloromethamphetamine (CMA), which is metabolized to para-chloroamphetamine in vivo, has neurotoxic properties as well. Conversely, the phentermine (α-methyl) analogue chlorphentermine does not appear to be neurotoxic.[12]
Pharmacology
[edit]PCA acts as a serotonin, norepinephrine, and dopamine releasing agent (SNDRA).[13][14][15] Its EC50 values for monoamine release are 68.5 nM for dopamine and 23.5 nM for norepinephrine, whereas its EC50 value for serotonin release was not reported.[13][14]
PCA does not show effects like those of the selective norepinephrine and dopamine releasing agent (NDRA) amphetamine in animals but instead fully substitutes for other serotonin releasing agents like (+)-MBDB and MMAI.[15] The findings with PCA are in contrast to those with para-fluoroamphetamine, which acts as a selective NDRA similarly to amphetamine,[16] fully substitutes for amphetamine in animals, and fails to substitute for (+)-MBDB or MMAI.[15]
Legal status
[edit]China
[edit]As of October 2015, 4-CA is a controlled substance in China.[17]
See also
[edit]- para-Chloromethamphetamine (4-CMA)
- 3,4-Dichloroamphetamine (3,4-DCA)
- 2,4-Dichloroamphetamine (2,4-DCA)
- 3-Chloroamphetamine (3-CA)
- 4-Fluoroamphetamine (4-FA)
- 4-Methylamphetamine (4-MA)
- 5,7-Dihydroxytryptamine (5,7-DHT)
- para-Bromoamphetamine (PBA)
- para-Iodoamphetamine (PIA)
References
[edit]- ^ Miller KJ, Anderholm DC, Ames MM (May 1986). "Metabolic activation of the serotonergic neurotoxin para-chloroamphetamine to chemically reactive intermediates by hepatic and brain microsomal preparations". Biochemical Pharmacology. 35 (10): 1737–1742. doi:10.1016/0006-2952(86)90332-1. PMID 3707603.
- ^ Gal EM, Cristiansen PA, Yunger LM (January 1975). "Effect of p-chloroamphetamine on cerebral tryptophan-5-hydroxylase in vivo: a reexamination". Neuropharmacology. 14 (1): 31–39. doi:10.1016/0028-3908(75)90063-5. PMID 125387. S2CID 1068793.
- ^ Curzon G, Fernando JC, Marsden CA (August 1978). "5-Hydroxytryptamine: the effects of impaired synthesis on its metabolism and release in rat". British Journal of Pharmacology. 63 (4): 627–634. doi:10.1111/j.1476-5381.1978.tb17275.x. PMC 1668117. PMID 80243.
- ^ Colado MI, Murray TK, Green AR (March 1993). "5-HT loss in rat brain following 3,4-methylenedioxymethamphetamine (MDMA), p-chloroamphetamine and fenfluramine administration and effects of chlormethiazole and dizocilpine". British Journal of Pharmacology. 108 (3): 583–589. doi:10.1111/j.1476-5381.1993.tb12846.x. PMC 1908028. PMID 7682129.
- ^ Freo U, Pietrini P, Pizzolato G, Furey M, Merico A, Ruggero S, et al. (November 1995). "Cerebral metabolic responses to clomipramine are greatly reduced following pretreatment with the specific serotonin neurotoxin para-chloroamphetamine (PCA). A 2-deoxyglucose study in rats". Neuropsychopharmacology. 13 (3): 215–222. doi:10.1016/0893-133X(95)00053-G. PMID 8602894.
- ^ Lin TC, Lin DL, Lua AC (May 2011). "Detection of p-chloroamphetamine in urine samples with mass spectrometry". Journal of Analytical Toxicology. 35 (4): 205–210. doi:10.1093/anatox/35.4.205. PMID 21513613.
- ^ Fuller RW, Schaffer RJ, Roush BW, Molloy BB (May 1972). "Drug disposition as a factor in the lowering of brain serotonin by chloroamphetamines in the rat". Biochemical Pharmacology. 21 (10): 1413–1417. doi:10.1016/0006-2952(72)90365-6. PMID 5029422.
- ^ Ogren SO, Ross SB (October 1977). "Substituted amphetamine derivatives. II. Behavioural effects in mice related to monoaminergic neurones". Acta Pharmacologica et Toxicologica. 41 (4): 353–368. doi:10.1111/j.1600-0773.1977.tb02674.x. PMID 303437.
- ^ Ross SB, Kelder D (May 1979). "Inhibition of 3H-dopamine accumulation in reserpinized and normal rat striatum". Acta Pharmacologica et Toxicologica. 44 (5): 329–335. doi:10.1111/j.1600-0773.1979.tb02339.x. PMID 474143.
- ^ Fuller RW, Baker JC (November 1974). "Long-lasting reduction of brain 5-hydroxytryptamine concentration by 3-chloramphetamine and 4-chloroamphetamine in iprindole-treated rats". The Journal of Pharmacy and Pharmacology. 26 (11): 912–914. doi:10.1111/j.2042-7158.1974.tb09206.x. PMID 4156568. S2CID 41833796.
- ^ Ross SB, Ogren SO, Renyi AL (October 1977). "Substituted amphetamine derivatives. I. Effect on uptake and release of biogenic monoamines and on monoamine oxidase in the mouse brain". Acta Pharmacologica et Toxicologica. 41 (4): 337–352. doi:10.1111/j.1600-0773.1977.tb02673.x. PMID 579062.
- ^ Lovenberg W, Walker MN, Baumgarten HG (1976). "Chlorinated amphetamines: drugs or toxins". Clinical Pharmacology of Serotonin. Frontiers of Neurology and Neuroscience. Vol. 3. pp. 109–114. doi:10.1159/000399342. ISBN 978-3-8055-2328-8. PMID 790166.
A methylated analogue of p-chloroamphetamine is chlorphentermine (fig. 1). This compound is marketed as an appetite suppressant Pre-Sate® and it seemed of interest to reevaluate the effects of this compound on the serotonergic system. One day following the administration of 20 mg/kg to rats there appeared to be little loss of tryptophan hydroxylase in any of the brain regions; e.g., mesencephalic tegmentum 124 %, mesencephalic tectum 95.7 % and striatum 103.5 %, of control values. While this preliminary experiment would suggest that chlorphentermine is not neurotoxic, it would seem in view of the similarity of its structure to p-chloroamphetamine that considerably more detailed experiments should be done to evaluate the long-term effects of this drug and its potential neurotoxicity.
- ^ a b Forsyth, Andrea N (22 May 2012). "Synthesis and Biological Evaluation of Rigid Analogues of Methamphetamines". ScholarWorks@UNO. Retrieved 4 November 2024.
- ^ a b Blough B (July 2008). "Dopamine-releasing agents". Dopamine Transporters: Chemistry, Biology and Pharmacology. Hoboken [NJ]: Wiley. pp. 305–320. ISBN 978-0-470-11790-3. Archived from the original on 4 November 2024.
Aromatic substitution changes have a significant effect on amphetamine potencies. Chloro-, fluoro-, and methyl-substituted compounds were found to have dopamine-releasing potencies within an order of magnitude of that of amphetamine. EC50 values ranged from 11.8 nM for m-chloroamphetamine (20) to 68.2 nM for p-chloroamphetamine (16), with the exception of o-methylamphetamine (23), which was substantially weaker (EC50 = 127 nM). The meta-substituted compounds were found to be more potent than their corresponding ortho or para analogs. [...] TABLE 11-2 Comparison of the DAT- and NET-Releasing Activity of a Series of Amphetamines [...] Compound: 16 [PCA]. Structure: [...]. EC50 (nM): DAT: 68.5. NET: 23.5 nM.
- ^ a b c Marona-Lewicka D, Rhee GS, Sprague JE, Nichols DE (December 1995). "Psychostimulant-like effects of p-fluoroamphetamine in the rat". Eur J Pharmacol. 287 (2): 105–113. doi:10.1016/0014-2999(95)00478-5. PMID 8749023.
- ^ Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL (May 2005). "Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs". The Journal of Pharmacology and Experimental Therapeutics. 313 (2): 848–854. doi:10.1124/jpet.104.080101. PMID 15677348. S2CID 12135483.
- ^ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 1 October 2015.
